Symptom severity is more closely associated with social functioning status in inpatients with schizophrenia than cognitive deficits
2012-07-08SaiZUOLindaBYRNEDaihuiPENGDavidMELLORMaritaMcCABEJieZHANGJiaHUANGYifengXU
Sai ZUO, Linda K. BYRNE, Daihui PENG, David MELLOR, Marita McCABE, Jie ZHANG, Jia HUANG, Yifeng XU*
Symptom severity is more closely associated with social functioning status in inpatients with schizophrenia than cognitive deficits
Sai ZUO1, Linda K. BYRNE2*, Daihui PENG1, David MELLOR2, Marita McCABE2, Jie ZHANG1, Jia HUANG1, Yifeng XU1*
Background:Prior research has determined that impairment in neurocognition and psychiatric symptoms contribute to reduced occupational and social functioning in schizophrenia.
Objective:Evaluate the relationships of neurocognition, psychiatric symptoms, and psychosocial functioning in male inpatients with schizophrenia in China.
Methods:Fifty-one male patients currently hospitalised at the Shanghai Mental Health Center with a diagnosis of schizophrenia were recruited and 40 of them were included in the final analysis. Participants were assessed with Chinese versions of the Personal and Social Performance Scale (PSP), Clinical Global Impression-Severity (CGI-S) scale, Positive and Negative Symptom Scale (PANSS), Letter-Number Sequencing Task, and Hong Kong List Learning Test.
Results:Robust negative correlations were found between three clinical subscale scores derived from the PANSS and the global measures of social function (the total PSP score and the CGI-S score). Performance on the neurocognitive tasks was not associated with either symptoms or social functioning status.
Conclusions:Among inpatients in the acute phase of schizophrenia, the severity of the clinical symptoms—not the degree of the neurocognitive impairment—is closely associated with the level of social functioning.
1. Introduction
Neurocognitive deficits and the expression of symptoms in schizophrenia contribute to the chronic disability in psychological, social and occupational functioning experienced by sufferers.[1-3]The relative contribution of symptoms versus neurocognition is complex and still not fully understood. Some studies indicate the primacy of neurocognitive variables over symptoms[4]whereas others support a mediation model whereby symptoms at least partially mediate the relationship between neurocogntion and outcome.[5]
At least seven areas of neurocognitive functioning (speed of processing, attention/vigilance, working memory, verbal learning, visual learning, and reasoning and problem solving) are commonly impaired in schizophrenia.[6]Verbal memory and working memory deserve special consideration for neurobiological reasons and because of their relationship to functional outcome.[7]A 2004 review and meta-analysis by Green and colleagues[4]concluded that immediate (working) and secondary verbal memory are the most potent neurocognitive predictors of functional outcome. These findings have been confirmed by a more recent meta-analysis[5]and a large scale study of older patients with schizophrenia.[8]However, not all research has confirmed this relationship. For example, Addington and colleagues[9]found no significant associations between cognitive measures and social functioning. One study[10]found that secondary memory and verbal IQ correlated with some outcome measures, but immediate memory did not.
Functional outcome has been measured in a variety of ways including activities of daily living and general community functioning. It can also be assessed in terms of success in rehabilitation programs or in terms of general problem solving abilities. Hsieh and colleagues[11]recently studied the relationship between performance and neurocognition in a Taiwanese sample using a battery of neurocognitive variables and functional measures, including the Personal and Social Performance scale (PSP) and the Activities of Daily Living (ADL) scale. They found significant correlations between verbal memory performance and total PSPscore. Baseline measures of verbal memory have been found to predict functional outcomes from one month[12]up to seven years.[13]Current performance on these neurocognitive measures has also been found to be a strong predictor of functional disability in people with schizophrenia who are symptomatically stable;[14]this recent study found that working memory predicted work and educational functioning while verbal memory predicted skills at independent living.
Symptoms in schizophrenia also have a significant role to play in predicting functional outcomes. The three factor model of positive, negative and disorganised symptoms in schizophrenia is currently the most widely applied[15-17]although this structure is not universally accepted.[18]Recent studies emphasize the role of negative symptoms as predictors of poorer social and occupational outcomes.[5,13,14]At least one study found that disorganised symptoms predict social functioning.[19]There has been limited research support for the role of positive symptoms as predictors of functional outcomes, though one older study[20]reported that baseline levels of positive symptoms—especially thought disturbance—predicted poor outcomes for patients with chronic schizophrenia.
The relationship between symptoms and neurocognition is complex. Generally, in cross-sectional studies negative symptoms have produced the strongest correlations with neurocognitive deficits.[5]Prominent negative symptoms have been linked to poorer performance on verbal memory tasks[21]and on verbal working memory.[22]There is some support for the role of disorganised thinking or behaviour in impairing neurocognitive performance, particularly on complex executive processes such as working memory.[23]Less convincing is the evidence that positive symptoms are related to impaired neurocognitive abilities. One study found increased perseverative errors and a bias towards false alarms on a list learning task,[24]but a recent metaanalysis[5]found virtually no relationship.
The aim of the current study is to investigate the relationships among clinical, cognitive and functional variables in a sample of Chinese inpatients with schizophrenia. We were particularly interested in examining the relationship between these variables in this population before they were clinically stable as most work to date has examined how baseline neurocognition and symptoms predict future functioning. We hypothesized that measures of social functioning would be more strongly correlated to cognitive measures of verbal memory and verbal working memory than to measures of psychiatric symptoms. Further, we hypothesized that the measures of psychomotor poverty (negative) symptoms would be more strongly correlated to cognitive performance than measures of positive symptoms or disorganized symptoms.
2. Methods
2.1 Participants
The enrolment of participants is shown in Figure 1. The study was conducted at two acute-care male wardsof the Shanghai Mental Health Center, one ward has 50 beds with an average stay of 45 days and the other has 90 beds with an average stay of 60 days. Patients were eligible to participate in the study if they were treated on one of the two wards from October 2010 to January 2011, were 18-55 years of age, had a current DSM-IV diagnosis of Schizophrenia or Schizoaffective Disorder, and did not have a co-morbid substance use disorder. As shown in Figure 1, 40 of the 54 eligible patients completed the assessments of their clinical, cognitive and functional status. Their characteristics are presented in Table 1. They were primarily middleage males who had, on average, been ill for more than 20 years. All 40 patients were being treated with antipsychotic medication during the time of the assessment (35 on atypical antipsychotics, 1 on typical antipsychotics, and 4 on a combination of typical and atypical antipsychotics), and were clinically stable (i.e., not actively psychotic).
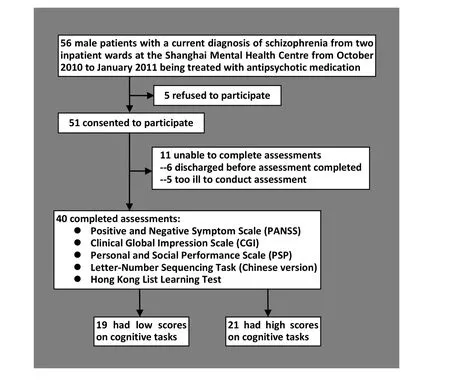
Figure 1. Flowchart of enrolment in the study
All participants provided written informed consent. The study received institutional ethics approval from Deakin University and from the Shanghai Mental Health Center.
2.2 Assessments
2.2.1 Clinical Symptoms and Social Functioning
All participants were rated on three clinical scales measuring symptoms, global functioning, and social functioning by one of two experienced psychiatrists (JZ, JH) trained in the consensus ratings of these measures.
A Chinese version of the Positive and Negative Symptom Scales (PANSS)[25]was administered to all participants. The PANSS items were subdivided into three syndromes (reality distortion [5 items], psychomotor poverty [7 items] and disorganised [4 items]) based on Liddle’s 1987[15]description of these three syndromes of schizophrenia. This method of categorizing symptoms was chosen because it has been supported by factor analysis of the PANSS[15,26,27]and been used in other studies examining neurocognition and symptoms in schizophrenia.[25]In addition to the Liddle syndrome scores, we computed the total PANSS score (based on all 30 items) according to the PANSS manual to allow comparison with other studies that have examined the reliability and validity of the Chinese version of the PANSS.[11,28]
The Clinical Global Impression Scale (CGI) is a widely used tool of global functioning that yields three measures; severity of illness, global improvement and an efficacy index. It is widely used in China.[29,30]The Severity of Illness (CGI-S) item requires clinicians to rate the severity of participants’ illness at the time of assessment on a 7-point scale, from 1 (normal, not at all ill) to 7 (among the most severely ill).

Table 1. Demographic, clinical and cognitive characteristics of 40 male inpatients with schizophrenia included in the study
The Personal and Social Performance Scale (PSP)[31]uses a 100-point rating scale to assesses four domains of social functioning including (a) socially useful activities, such as work and study; (b) personal and social relationships; (c) self-care; and (d) disturbing and aggressive behaviours. The total score, which is a composite measure of the four domains, is divided into three levels: a score of 71-100 indicates mild difficulties across the domains; 31-70 indicates varying degrees of impairment and disability; and 0-30 indicates poor functioning that requires intensive support or supervision. Assessment of a Chinese version of the PSP[28]in a combined sample of inpatients and outpatients demonstrated robust construct validity and good internal consistency (Cronbach’s alpha=0.84), testretest reliability (intraclass correlation coefficient=0.95) and inter-rater reliability (kappa value=0.82). However, the PSP has not previously been used exclusively with an inpatient sample.
2.2.2 Cognitive assessments
Cognitive assessments were brief; they included a measure of verbal working memory and a measure of verbal memory and learning. The letter-number sequencing task has established credentials and is a robust, yet quick and easily administered test of verbal working memory.[32]A Chinese version of this task[33]was used in the current study. The longest sequence achieved and total score were recorded. The longest sequence achieved was chosen as the variable of interest that was employed in the subsequent analysis as it best reflects working memory capacity.
The verbal memory task employed was Form B of the Hong Kong List Learning Test,[34]a Chinese verbal learning and memory test that has been well validated in a variety of populations including people with schizophrenia.[35]Form B has 16 words from four categories: clothing and accessories, music, flowers, and occupations. Participants are informed about the four categories and about the total number of items before the names of the items are presented. After the researcher reads the words, participants are asked to recall as many of the items as possible (in any order). The total score for the test is used as the measure of verbal memory in the subsequent analysis.
These cognitive tasks were administered by a trained research assistant and took between 10 and 15 minutes to complete.
2.3 Statistical methods
The relationship of the three different types of measures—clinical, cognitive, and functional—was assessed using Pearson correlation coefficients. The internal consistency of the various measures was assessed using Cronbach’s alpha. The chlorpromazineequivalent daily dosage of the participants was not normally distributed so a log transformation of this variable was used in the statistical analyses.
To assess whether or not the relationship of cognitive functioning with social functioning varied for persons with different levels of impairment, we conducted a post-hoc analysis by splitting the sample based on whether they were 'high' or 'low' in cognitive functioning. This was achieved by forming a composite cognitive z-score for each participant from the two cognitive measures (LNS and HKLT) and then classifying the 21 patients with a positive composite z-score to the‘high’ cognitive functioning group and the 19 patients with a negative composite z-score to the ‘low’ cognitive functioning group.
3. Results
Internal consistency of the three factor scores derived from the PANSS used in this analysis were as follows: reality distortion alpha=0.83, psychomotor poverty alpha=0.90; and disorganisation alpha=0.48. The internal consistency of the total PANSS score was 0.915. Internal consistency of the overall PSP score using Cronbach’s alpha was 0.77.
Table 2 presents the bivariate Pearson’s product moment correlations of the symptom and cognitive variables with the PSP and CGI. Also included are possible confounding variables such as years of education and the log transformed medication dosage variable. Spearman rho correlations produced a similar pattern so they are not reported here. Given that there are a large number of correlations considered, the level of statistical significance was set at 0.01 rather 0.05 to reduce the risk of Type 1 error.
The two cognitive variables (the Letter-Numbering Sequencing task and the Hong Kong List Learning Test) were not significantly related to any of the clinical or social functioning variables but they were positively related to the educational level of the participants. There was, however, a non-significant trend association between the working memory variable (LNS) and the disturbing or aggressive behaviour PSP subscale score (r=0.33, p=0.038). The two global functioning measures (CGI-S and the PSP total score) were both closely correlated with the three clinical measures derived from the PANSS (r-values all greater than 0.50) and strongly correlated with the overall PANSS score (r= -0.73, p<0.001). The score of the PSP subscale on personal and social relationships was also closely related to all three clinical measures. But the scores of the PSP subscales on socially useful activities and self-care were only significantly related to the PANSS psychomotor poverty measure and the score of the PSP subscale on disturbing or aggressive behaviour was not significantly related to any of the clinical measures (though there was a trend correlation with all three clinical measures).
We conducted a stratified post-hoc analysis (dividing the sample into those with high cognitive functioningand low cognitive functioning) to assess whether or not the failure to identify a relationship between the cognitive measures and the functioning measures was due to the admixture of patients with widely differing cognitive states. No statistically significant relationships were found in the correlation analysis of cognitive measures with social functioning measures in either the high cognitive functioning group or the low cognitivefunctioning group, though the relationship between the LNS score and the disturbing/aggressive PSP subscale score in the low cognitive functioning group was significant at a trend level (r=0.47, p=0.042). Moreover, there were no statistically significant differences between the high and low cognitive functioning groups in the magnitude of the correlation coefficients for the cognitive and functioning measures. (Data not presented).
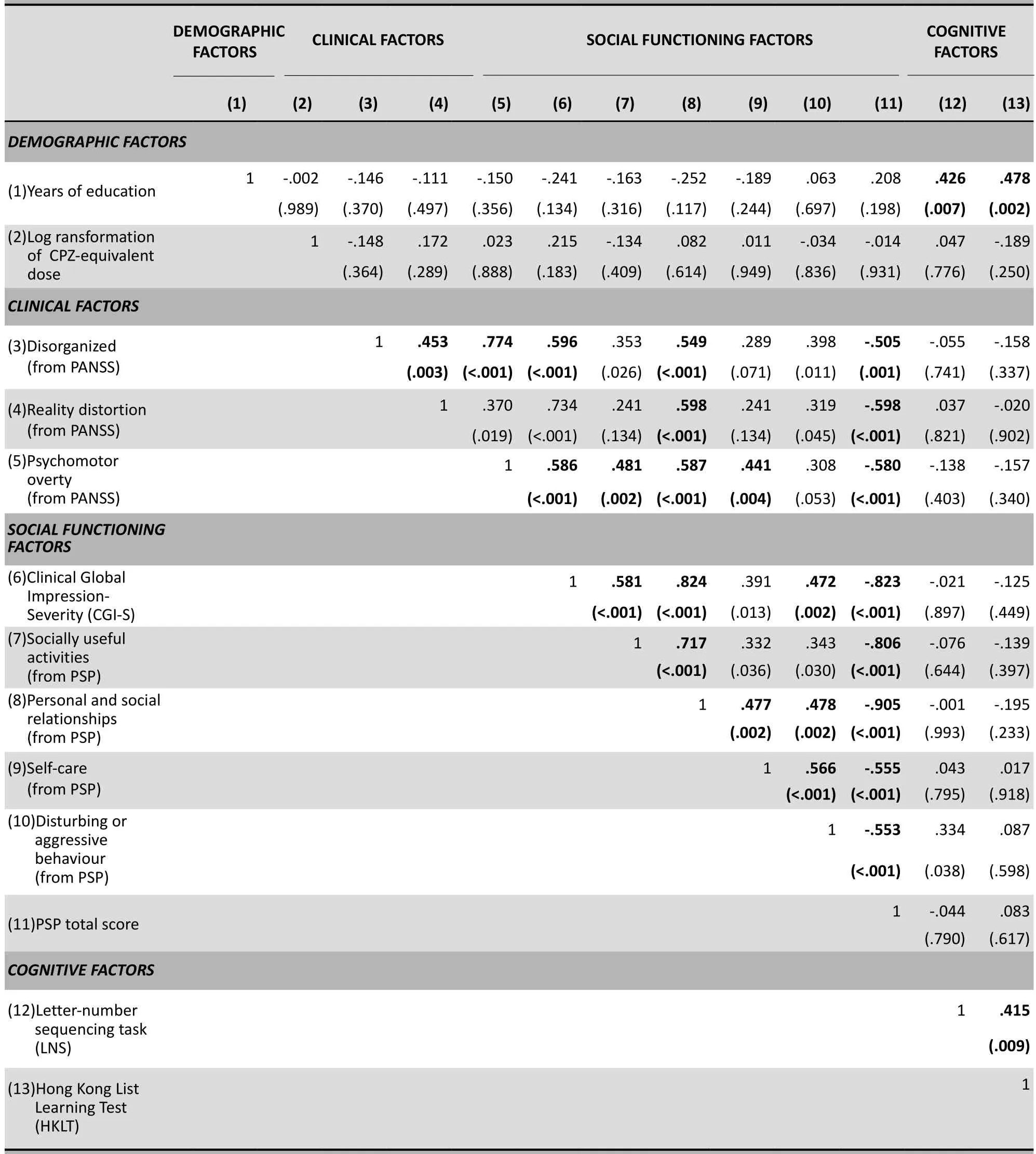
Table 2. Pearson correlations and associated p-values of clinical, cognitive and social functioning variables in 40 inpatients with schizophreniaa
4. Discussion
4.1 Main Findings
Our original hypotheses were not confirmed. In this sample of male inpatients with schizophrenia we found little evidence of a relationship between neurocognitive variables and either symptoms or functional measures, with the exception of a nonsignificant trend relationship between decrements in verbal working memory performance and increased scores on the disturbing or aggressive behaviour subscale of the PSP. These findings are out of step with most prevailing literature in the area which has consistently found that verbal memory and, to a lesser extent, working memory are associated with functioning.[4]Our findings are, however, similar to the early findings of Addington and colleagues[9]who found no relationship between neurocognition and functioning (as assessed by the social dysfunction index and the social adjustment scale). They hypothesized that their negative findings were the result of specific characteristics of their sample; given the high level of impairment of the patients in their sample, they suggested that the relationship between neurocogntive variables and functional outcome may only hold true for higher functioning individuals. To assess this possibility we conducted a post-hoc analysis of our data dividing the sample into two groups, one group with relatively high cognitive functioning and one with relatively low cognitive functioning. We found no significant relationships between our cognitive measures (LNS and HKLT) and the social functioning PSP measures in either of the groups, so our results do not support Addington’s hypothesis.
We did, however, find robust correlations between all three symptom domains derived from the PANSS and our global functioning measures (CGI-S and overall PSP score). These correlations were substantially stronger than those reported by Patrick and colleagues;[36]they reported a correlation of PSP total score and PANSS total score of -0.32, versus the -0.73 correlation found in the current study. This difference may have occurred because our sample was less symptomatic at the time of assessment; unlike Patrick and colleagues we did not require elevated PANSS scores for inclusion in the study. In our study the personal and social relationships subscale of the PSP was also closely associated with all three symptom domains. The relationship between the psychomotor poverty dimension of the PANSS and the personal and social relationships subscale of the PSP mirrors the findings of others.[37]
4.2 Limitations
There were a number of limitations to our study. The modest sample size reduced the power to detect statistical significance. The large number of correlations presented in Table 2 (78 correlation coefficients) increased the possibility of Type I errors (i.e., inappropriately considering a chance difference as statistically significant). The three-factor model of the PANSS used in the study has not been validated in China and the internal consistency of the disorganization dimension of the PANSS was weak (alpha=0.48). Several of the items in the PSP related to social functioning were difficult to assess reliably in the inpatient setting; this may have resulted in a restriction in the range of scores of the related subscales. Only two relatively simple measures of neurocognitive functioning were included; a more comprehensive assessment would have provided more convincing proof of our finding of a lack of relationship between cognitive function and social function. The mean duration of illness of the sample was 23 years; the results may have been different for a less chronic sample. Finally, this is a cross-sectional study so we are only able to evaluate associations, not the cause-effect relationship of the variables considered.
4.3 Implications
The findings presented here add to the substantial body of work examining the relationships among neurocognition, symptoms and functional status in schizophrenia. We failed to replicate what many others have found in regard to a strong relationship between neurocognition and functional status. We also failed to replicate the common finding of a relationship between increased negative symptoms and poorer cognitive functioning. It may be that this is the result of some limitations in the study such as too few neurocognitive variables or a small sample size. However, it may also be an indication that the extent to which neurocognition influences the social functioning of an individual is moderated by the severity of their psychological symptoms and the setting in which they are assessed. It appears, at least in this sample, that when individuals with schizophrenia are suffering from acute symptoms that require hospitalisation it is the symptoms, not neurocognitive variables, that are more important to personal and social functioning. Perhaps neurocognitive factors become more important as symptoms resolve. To test this idea one would need to follow patients over time and determine whether or not the relativestrength of the relationship between cognitive measures and personal and social functioning increases as the severity of clinical symptoms decreases.
Conflict of Interest
The authors report no conflict of interest related to this paper.
Funding
This research was supported by funds awarded to Dr. Linda Byrne from the Adult Mental Health Strategic Research Centre and the School of Psychology at Deakin University, Melbourne, Australia and Dr. Daihui Peng from the Shanghai City Health Bureau Fund (grant number: 2010086).
1. Couture SM, Penn DL, Roberts DL.The functional significance of social cognition in schizophrenia: a review. Schizophr Bull 2006; 32 (Suppl 1): S44-63.
2. Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for Matrics. Schizophr Res 2004;72(1): 41-51.
3. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 1998; 12(3):426-445.
4. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the right stuff? Schizophr Bull 2000;26(1):119-135.
5. Ventura J, Helleman GS, Thames AD, Koellner V,Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res 2009;113(2-3):189-199.
6. Nuechterlein KF, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in Schizophrenia. Schizophr Res 2004;72(1):29-39.
7. Goldberg, Green. Neurocognitive deficits in schizophrenia. In Davis, Chamey, Coyle, Nemeroff (eds). Neuropsychopharmacology: The Fifth Generation of Progress. American College of Neuropsychopharmacology, 2002:657-668.
8. Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, et al. Predicting schizophrenia patients’ real world behaviour with specific neuropsychological and functional capacity measures. Biol Psychiatry 2008; 63(5):505-511.
9. Addington J, McCleary L,Munroe-Blum H. Relationship between cognitive and social dysfunction in schizophrenia. Schizophr Res 1998;34(1-2): 59-66.
10. Bellack AS, Sayers M, Mueser K, Bennett M. Evaluation of social problem solving in schizophrenia. J Abnorm Psychol 1994;103(2):371-378.
11. Hsieh PC, Huang HY, Wang HC, Liu YC, Bai YM, Chen KC, et al. Intercorrelations between the Personal and Social Performance Scale, cognitive function, and activities of daily living. J Nerv Ment Dis 2001; 199(7): 513-515.
12. Schuepbach D, Keshavan MS, Kmiec JA,Sweeney JA. Negative symptom resolution and improvements in specific cognitive deficits after acute treatment in first-episode schizophrenia. Schizophr Res 2002;53(3):249-261.
13. Milev P, Beng-Choon H, Arndt S, Andreasen NC. Predictive
values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry 2005;162(3):495-506.
14. Shamsi S, Lau A, Lencz T, Burdick KE, DeRosse P, Brenner R, et al. Cognitive and symptomatic predictors of functional disability in schizophrenia. Schizophr Res 2011;126(1-3): 257-264.
15. Liddle PF. Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med 1987; 17(1): 49-57.
16. Toomey R, Kremen WS, Simpson JC, Samson JA, Seidman LJ, Lyons MJ, et al. Revisiting the factor structure for positive and negative symptoms: evidence from a large heterogeneous group of psychiatric patients. Am J Psychiatry 1997, 154(3): 371-377.
17. Malla AK, Norman RM, Aguilar O, Cortese L. Relationship between neurological 'soft signs' and syndromes of schizophrenia. Acta Psychiatr Scand 1997;96(4):274-280.
18. Stuart GW, Pantelis C, Klimidis S, Minas IH. The three-syndrome model of schizophrenia: Meta-analysis of an artefact. Schizophr Res 1999;39(3):233-242.
19. Brune M, Schaub D, Juckel G,Langdon R. Social skills and behavioural problems in schizophrenia: the role of mental state, attribution, neurocognition and clinical symptomatology. Psychiatry Res 2011; 190(1): 9-11.
20. Kay SR, Murrill LM. Predicting outcome of schizophrenia: significance of symptom profiles and outcome dimensions. Compr Psychiatry 1990;31(2): 91-102.
21. Stirling JD, Hellewell JS, Hewitt J. Verbal memory impairment in schizophrenia: no sparing of short-term recall. Schizophr Res 1997; 25(2):85-95.
22. Greenwood KE, Landau S, Wykes T. Negative symptoms and specific cognitive impairments as combined targets for improved functional outcome within cognitive remediation therapy. Schizophr Bull 2005;31(4): 910-921.
23. Cameron AM, Oram J, Geffen GM, Kavanagh DJ, McGrath JJ, Geffen LB. Working memory correlates of three symptom clusters in schizophrenia. Psychiatry Res 2002; 110(1): 49-61.
24. Brebion G, Smith MJ, Amador X, Malaspina D, Gorman JM. Clinical correlates of memory in schizophrenia: differential links between depression, positive and negative symptoms and two types of memory impairment. Am J Psychiatry 1997; 154(11): 1538-1543.
25. He YL, Zhang MY. The positive and negative syndrome scale (PANSS) and its application. Journal of Clinical Psychiatry 1997;7(6):353-355. (in Chinese)
26. Kay SR, Sevy S. Pyramidal model of schizophrenia. Schizophr Bull 1990; 16(3): 537-545.
27. Mass R, Schömig T, Hitchfeld K, Wall E, Haansen C. Psychopathological syndromes of schizophrenia. Schizophr Bull 2000; 26(1): 167-177.
28. Tianmei S, Liang S, Yun'ai S, Chenghua T, Jun Y, Jia C, et al.The Chinese version of the personal and social performance scale (PSP): validity and reliability. Psychiatry Res 2010;185(1-2):275-279.
29. Liu J, Guo YQ, Yang XL. Efficacy and tolerability of olanzapine on childhood-onset schizophrenia. Chinese Mental Health Journal 2005;19(2): 71-81. (in Chinese)
30. Zhang ZJ, Kang WH, Li Q, Wang XY, Yao SM, Ma AQ. Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: A double-blind, randomized, placebo-controlled study. Schizophr Res 2006;88(1-3):102-110.
31. Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 2000;101(4):323-329.
32. Gold JM, Carpenter CJ, Randolph C, Goldberg TE, Weinberger DE. Auditory working memory and Wisconsin card sorting performance in schizophrenia.Arch Gen Psychiatry1997;54(2):159-165.
33. Chan RC, Wang Y, Deng Y, Zhang Y, Yiao X, Zhang C. The development of a chinese equivalence version of the letternumber span test.Clin Neuropsychol2008;22(1):112-121.
34. Chan AS.The Hong Kong List Learning Test.2nd ed. Chinese University of Hong Kong: Hong Kong.2006.
35. Chan AS, Kwok IC, Chiu H, Lam L, Pang A, Chow L. Memory and organizational strategies in chronic and acute schizophrenia patients.Schizophr Res2000;41(3):431-445.
36. Patrick DL, Burns T, Morisini P, Rothman B, Gagnon DD,Wild D, et al. Reliability, validity and ability to detect change of the clinician-rated Personal and Social Performance scale inpatients with acute symptoms of schizophrenia.Curr Med Res Opin2009;25(2):325-338.
37. Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms.Am J Psychiatry2006;163(3): 418-425.
2012-02-08; accepted:2012-03-28)
10.3969/j.issn.1002-0829.2012.02.003
1Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
2School of Psychology, Deakin University, Melbourne, Australia
*Joint corresponding authors: Linda K. BYRNE, linda.byrne@deakin.edu.au; Yifeng XU, hyyyyb@gmail.com
杂志排行
上海精神医学的其它文章
- Hemispheric dominance during the mental rotation task in patients with schizophrenia
- Correlation between insight and internalized stigma in patients with schizophrenia
- Cross-sectional survey of prevalence and personality characteristics of college students with internet addiction in Wenzhou, China
- Latent variable modeling
- · In this issue ·
- Research in China on event-related potentials in patients with schizophrenia
