Effects of Subchronic Aluminum Exposure on Amino Acids Neurotransmitters in Chicken Brain
2012-07-02HanYanfeiXiaShiliangBaiChongshengZhangJihongandLiYanfei
Han Yan-fei, Xia Shi-liang, Bai Chong-sheng, Zhang Ji-hong, and Li Yan-fei
College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
Introduction
Aluminum (Al)is the most abundant metallic element on the earth. It is used widely because of its excellent properties. With the development of Al industry and the occurrence of acid rains, Al poisoning happen more often than ever. The dosage of Al exposed to animal organism has increased greatly, and Al caused central nervous disorders. Excessive accumulation of Al may be one reason of senile dementia. The mechanism of Al neurotoxicity is inconclusive at present. However,research on the effects of Al on poultry amino acid neurotransmitters is scarce. This study was conducted by continuous peritoneal injection of different concentrations of the gradient the of aluminium trichloride(AlCl3). The aim of this study was to assess the effects of Al exposure on amino acid neurotransmitters. This study provided theoretical foundation for avoiding Al poisoning.
Materials and Methods
Animals and treatment
A total of 60 1-week-old Hyline White healthy chickens were acclimatized for 1 week and divided randomly into control group (GC), low-dose group (GL), middledose group (GM)and high-dose group (GH). And the dose of Al3+were 0, 18.31, 27.47, 36.62 mg · kg-1body weight per day, respectively. The chickens in Al-treated groups were injected with 0.2 mL sterile aqueous solution of AlCl3by intraperitoneal injection,GC with 0.2 mL sterile saline. All the breeding tools did not contain Al. The physical conditions of all chickens were observed and recorded in time, and the experiment lasted for 60 days.
Tissue sampling and processing
The chickens in each group were sacrif i ced by collecting blood from the heart at the end of experiment. The serum was separated automaticly at room temperature,and was stored at -20℃ to determine Al levels.
All chicken brains were peeled off in ice promptly.Twenty times weight perchloric acid (0.4 mol · L-1,4℃)were added and homogenized in ice-water.After the homogenate had been precipitated for 30 min and centrifuged at 10 000 r · min-1for 10 min, the supernatant was taken and stored at -70℃ for the determination of Al and neurotransmitter levels.
Determinion of Al levels in serum and brain
1.0 mL of serum and 1.0 mg of brain tissue were digested and Al levels were determined by flame atomic absorption spectrometry (Gu et al., 2009).
Determinion of neurotransmitters in chicken brain
The levels of amino acid neurotransmitters in chicken brain were determined by HPLC (Gu et al., 1995).
Data processing and analysis
Data were expressed as mean±standard deviation (SD).The results were analyzed by SPSS 13.0 for Windows(SPSS Inc, Chicago, IL, USA).
Results
Manifestations of chicken poisoning
Through this test, the chickens in each Al-treated group had poor appetite, weight loss, drooping wings,hair disheveled, head drooping, and lethargy, but no one dead. The chickens in GC had no abnormal manifestations.
Al levels in serum and brain
The Al levels in serum and brain in Al-treated groups were significantly higher than those in GC (p<0.05;p<0.01), and there was a dose-response relationship effect.
Levels of amino acid neurotransmitters
With the increase of Al, Glu levels increased, but there was no signif i cant difference compared with GC(p>0.05).
The levels of Asp, Gly, GABA, and Tau in Altreated groups were signif i cantly higher than those in GC (Table 2).
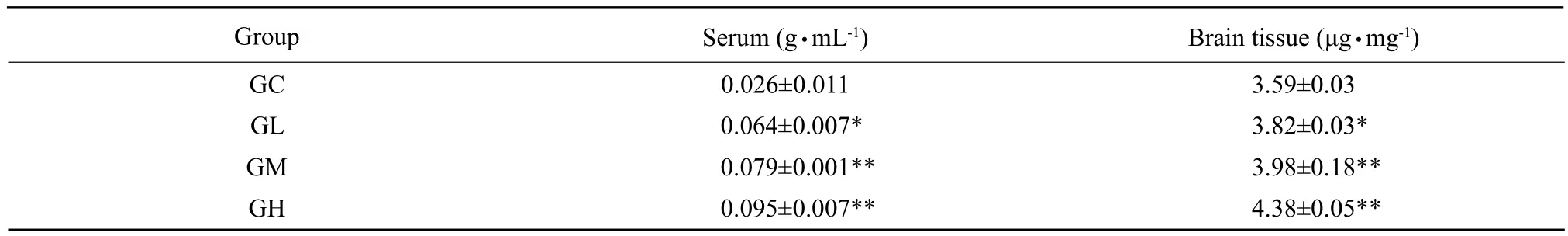
Table 1 Al levels in chicken serum and brain (mean±SD, n=10 per group)

Table 2 Levels of amino acid neurotransmitters in brain tissue (mmol • g-1, wet weight)
Discussion
To avoid the drawbacks of inaccurate exposure dose by drinking water, this test was conducted by intraperitoneal injection of different concentrations of gradient and fixed volume of AlCl3. Each Al-treated group appeared poor appetite, weight loss, lethargy,drooping wings, hair disheveled and head drooping after being exposed Al for 60 days. The Al levels in serum and brain tissue in Al-treated groups were signif i cantly higher than those in GC, which indicated that Al accumulated in chicken brain and induced neurotoxicity. Subchronic Al poisoning model had been built successfully.
Amino acid neurotransmitters are widely distributed in central nervous system, and are divided into excitatory amino acids (EAAs)and inhibitory amino acids (IAAs)according to its effects on postsynaptic neuron. Glutamic acid (Glu)and aspartic acid(Asp)are EAAs, and too much EAAs can cause its specific receptor's excessive continuous activation,which leads to neurotoxicity (such as N-methyl-D-aspartate (NMDA), etc). The overexcitation of NMDA receptors can cause continuous Ca2+inf l ux and delay damage of nerve cells. The continuous Ca2+influx leads to Ca2+accumulation in mitochondria, which causes damage of neuron. Then neural symptoms of lethargy and depression begin to appear (Leon et al.,2009). Neutral amino acid gamma amino butyric acid(GABA), taurine (Tau)and glycine (Gly)are IAAs,and play a compensatory role in inhibiting excitatory neurotoxicity in organism. In normal conditions, the EAAs and IAAs in brains are in dynamic balance,which plays an important part in sustaining the function of nervous system (Petroff, 2002). The changes of their levels are closely related to nervous system diseases.
Glu is important in nervous activities, but excessive or insufficient release will induce neurotoxicity.Currently, there are various reports about the effects of Al on EAAs levels. Li et al. (2008)fi lled stomach of Wistar rats with AlCl3, and found that Asp and Glu levels in brain tissue were significantly lower than those in the control. Nayak and Chatterje (2001)reported that Glu levels in rat cerebral cortex, thalamus, the midbrain-the hippocampus and cerebellum significantly increased after intraperitoneal injection of AlCl3for 4 weeks. After AlCl3had been added,Glu concentration in rats hippocampus increased by promoting the release of Glu neurotransmitters in vitro (Matyia, 2001). Studies have shown that Glu level between neurons signif i cantly increased after Al exposure, so was in synaptic cleft, which enhanced stimulation to postsynaptic membrane receptors,and resulted in neurotoxicity by increasing Ca2+concentration in neurons. All of these results indicated that neurotoxicity of Al3+connected with the increase of Glu which could change Ca2+concentration in cells(Guo and Liang, 2001). The test of Liu et al. (2008)showed that Ca2+concentration in neurons of chicken brain in Al-treated groups was significantly higher than that in the control. It showed that Al caused the increase of excitatory neurotransmitters, and aroused excessive sustained activation of NMDA receptors.The accumulation of intracellular Ca2+concentration might be one of the mechanisms that led to chicken excitotoxicity. Glu level in GH was higher than that in GC, while Asp level was signif i cantly higher than that in GC, which suggested that long-term Al exposure caused chicken excitotoxicity. The manifestations of depression and decreased activity were caused by neuron damage due to Al exposure. Therefore, Al might cause excitotoxicity as Glu receptor stimulant directly. In this research, Asp levels significantly increased, Glu levels did not change signif i cantly. The difference of the results might be related to exposure ways, exposure dosage, exposure time and different animal species. It might also be related to the complex biological functions, and the mechanism needed further study.
The study proved that GABA could reduce the release of Glu by presynaptic inhibition to relieve the toxic effects of EAAs. AlCl3could inhibit the accumulation and the transport of rat cranial nerve terminal particles on Glu and GABA in vitro, and Glu secretory increased significantly by stimulating rat stellate cells (Han and Wu, 1998). EAAs increased after cerebral ischemia, hypoxia, accompanied with the increase of IAAs release, which was a kind of protection against excessive excitement of EAAs.The increase of Tau and GABA levels were directly affected by positive regulation of Glu receptors (Oja and Saransaari, 2000). The results of this study showed that levels of Tau, GABA and Gly in GM and GH were signif i cantly higher than those in GC, and were consistent with previous studies, which was a kind of protection mechanism to excitatory neural toxicity for chickens. In conclusion, Asp, GABA, Tau and Gly increased in chicken brains after being exposed to Al,which caused excitability neural toxicity and showed neural inhibition.
Gu Q Y, Li X W, Zhang L C, et al. 2009. Effects of aluminium intoxication on metal elements levels of cerebrum and cerebellum in Chicks. China Poultry, 31(23): 15-17.
Gu Y J, Ni W, Bao W L, et al. 1995. Determinion of amino acids neurotransmitters with reversed-phase high performance liquid chromatography fl uorescence method. Journal of Shanghai Medical University, 22: 210-212.
Guo G W, Liang Y X. 2001. Aluminum-induced apoptosis in cultured astrocytes and its effect on calcium homeostasis. Brain Research,888(2): 221-226.
Han T Z, Wu F M. 1998. Neurobiology of learning and memory. Beijing Medical University and China Xiehe Medical University Joint Publishing, Beijing. pp. 254.
Leon R, Wu H, Jin Y, et al. 2009. Protective function of taurine in glutamate-induced apoptosis in cultured neurons. Neurosci Res, 87:1185-1194.
Li H Y, Liu P, Xu H L, et al. 2008. Effect of aluminum chelating agent on learning and memory and excitatory amino acids neurotransmitters in rats exposed to aluminum. Journal of Environment and Health,25(11): 960-962.
Liu H Q, Li Y F, Liu F T, et al. 2008. Effect of aluminum exposure on calcium homeostasis in chicken brain. China Poultry, 30(7): 18-21.
Matyia E. 2000. Aluminum enhances glutamate-mediated neurotoxicity in organotypic culture of rat hippocampus. Folia Neuropathol, 38(1):47-56.
Nayak P, Chatterje A K. 2001. Effects of aluminum exposure on brain glutamate and GABA systems: an experimental study in rats. Food Chem Toxico1, 39: 1285-1289.
Oja S S, Saransaari P. 2000. Modulation of taurine release by glutamate receptors and nitric oxide. Prog Neurobiol, 62: 407-425.
Petroff O A. 2002. GABA and gutamate in the human brain.Neuroscientist, 8(6): 562-573.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Establishment of TaqMan Real-time Quantitative PCR Assay for Foreign Gene Copy Numbers in Transgenic Soybean
- Study on Semen Freezing Preservation of German Shepherd Dogs
- In Vitro Study on Commercial Organic Acid Activate WD (WD)Against Four Pathogenic Bacteria
- Response of Osmotic Adjustment of Lactobacillus bulgaricus to NaCl Stress
- Temperature Simulation of Berry Slices Under Microwave Vacuum Puff i ng Conditions
- Storage System Design Scheme in Virtualization Construction
