Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
2012-03-22XIONGChunhua熊春华ZHUJingfei朱京妃SHENChen沈忱andCHENQing陈青
XIONG Chunhua (熊春华)**, ZHU Jingfei (朱京妃), SHEN Chen (沈忱) and CHEN Qing (陈青)
Department of Applied Chemistry, Zhejiang Gongshang University, Hangzhou 310012, China
1 INTRODUCTION
During the past decades, lanthanide elements have received great attention owing to their unique properties and wide applications. Praseodymium and its compounds have numerous industrial applications and they are currently used in ceramic industry,atomic batteries, photo catalytically active material and metallurgy [1-3]. The consumption of the materials is increasing with the rapid development of the modern industry in the world. Praseodymium may enter the environment in large quantities and accumulate in the human body via the food chain [4].
A number of methods including co-precipitation,solvent extraction, ion exchange and solid phase extraction have been employed for the removal of lanthanide elements from industrial effluents [5, 6]. Solvent extraction and ion exchange are the two most common methodologies for the preconcentration and separation of trace elements. However, the main drawback of solvent extraction process is the loss of extractant into the aqueous solution, which may cause environmental hazards and economic limitations. Compared with solvent extraction, ion exchange is simpler to operate and easier to separate. Various adsorbents including chelate resins and ion exchange resins are used in extraction of praseodymium ions [7, 8]. Chelate resins present good features of easy-functional and chemical stability,and shortcomings of poor hydrophilicity, slow adsorption rate and bad elution. Ion exchange resins are solid and suitably insolubilized high molecular weight polyelectrolytes. The resulting ion exchange is reversible and stoichiometric with the displacement of one ionic species by another on the exchanger [9, 10].
El-Dessouky et al. [11] studied the sorption of praseodymium (III) from nitrate medium using TVEXPHOR resin. However, the literature data concerning the adsorption process of praseodymium (III) onto strong acid ion exchange resin are limited. In this study, a strong acid ion exchange resin (D72) is used, which is a polymeric material containing a functional group(SO3H). It presents remarkable chemical and physical stability for temperatures of interest in treating aqueous solutions, and the functional groups ensure a large pH domain of work. Its principal characteristics are high exchange capacity and good ability of regeneration. Moreover, it is cheaper than imported resins.
In this work, the adsorption behavior and mechanism of Pr (III) on D72 resin are investigated with various chemical methods and IR spectrometry. Some factors affecting the adsorption, such as initial pH of solution, contact time and temperature, are examined.Adsorption experiments for kinetics and isotherm are carried out. Thermodynamic parameters of adsorption for Pr (III) are calculated. The experimental results may provide a path for the preconcentration and recovery of Pr (III) from aqueous solutions in the environmental protection and hydrometallurgical systems.
2 MATERIALS AND METHOD
2.1 Apparatus
Pr (III) was determined with Shimadzu UV-2550 UV-visible spectrophotometer. The resin dosage was measured by electronic balance of Sartorius BS 224S.Mettler Toledo delta 320 pH meter was used for pH measurement. The sample was shaken in the DSHZ-300 A temperature constant shaking machine. The water used in the present work was purified using Molresearch analysis-type ultra-pure water machine. The sample for IR spectroscopy was described by Nicolet 380 FT-IR.
2.2 Materials
D72 resin was supplied by Nankai University and its properties are shown in Table 1. Standard solutions of Pr(III) were prepared from Pr2O3(AR). HAc-NaAc with pH 3.00-6.00 and triethanolamine-nitric acid with pH 7.20 buffer solutions were prepared from the NaAc, HAc, N(C2H4OH)3and HNO3solutions. The chromophoric reagent of 0.1% arsenazo-I solution was obtained by dissolving 0.1000 g arsenazo-I powder into 100 ml deionized water. All other chemicals were of analytical grade and purified water was used throughout.
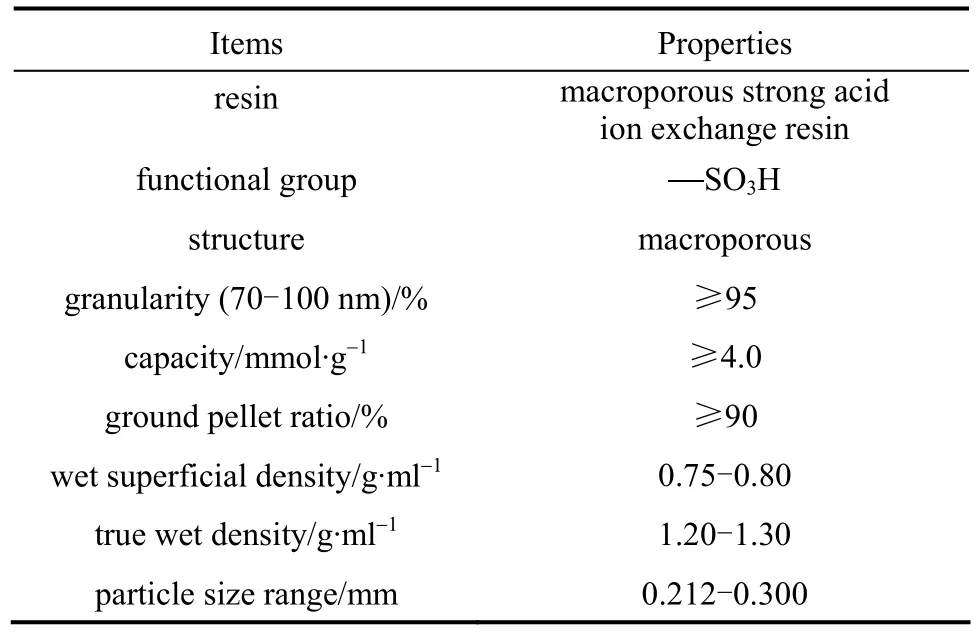
Table 1 General description and properties of D72
2.3 Adsorption experiments
2.3.1Batch studies
A desired amount of treated D72 resin was weighed and added into a conical flask, in which a desired volume of buffer solution with pH 3.00 was added. After 24 h, a required amount of standard solution of Pr (III) was added. The flask was shaken in a shaker at the constant temperature. Aliquot samples were taken from the flask at appropriate time intervals as necessary. The residual concentration of Pr (III) in the aqueous phases was measured at 580 nm.
2.3.2Desorption studies
Desorption of Pr (III) was performed by mixing D72-Pr (III) complexes and HCl-NaCl eluent solution of different concentrations, and shaken at 100 r·min-1for 24 h at 298K. The final Pr (III) concentrations in the aqueous phase were analyzed.
2.3.3Column studies
The fixed-bed experiments were carried out in glass columns (4.5 mm×235 mm) wet-packed with 300.0 mg (dry mass) D72 resin. The aqueous solution with known concentrations of Pr (III) was then fed to the top of the bed at 0.152 ml·min-1until the breakthrough curve was completed. The samples in the outlet were taken at the preset time intervals and the concentrations of Pr (III) were determined. In addition,dynamic desorption procedures were also carried out.With respect to the stripping of Pr (III) from the resin,eluent of 1.00 mol·L-1HCl-0.50 mol·L-1NaCl solution was employed.
2.3.4Analytical method
A solution containing Pr (III) was accurately added into a 25 ml colorimetric tube, and then 1 ml chromophoric reagent of 0.1% arsenazo-I solution and 10 ml pH 7.2 C6H15O3N-HNO3buffer solution were added. After the addition of purified water to the mark of colorimetric tube, the absorbency was determined in a 1 cm colorimetric vessel at 580 nm and compared with the blank test.
The adsorption capacity (Q), distribution coefficient (D) and desorption ratio (E) were calculated with the following formulas [12]:
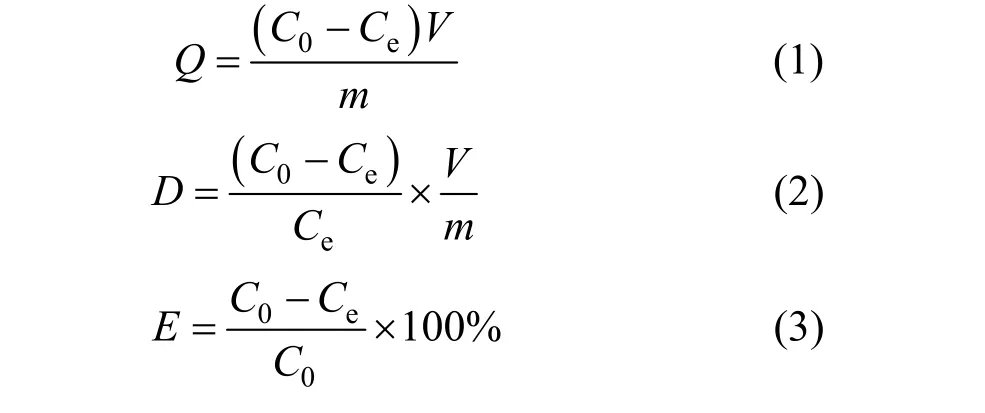
3 RESULTS AND DISCUSSION
3.1 Effect of pH on the adsorption for Pr (III)
The adsorption of Pr (III) from aqueous solutions onto D72 resin is primarily effected by the surface charge of the adsorbent [13]. The effect of pH on the adsorption behavior of D72 resin was tested with Pr(III) in the range of pH 2.6-5.0 for an initial concentration of Pr (III) 0.286 mg·ml-1at 298 K, 100 r·min-1.Fig. 1 shows that the adsorption capacity of D72 resin decreases evidently as the pH of solution increases and the maximum adsorption (1.64 mmol·g-1) is obtained at pH value of 3.0. Therefore, subsequent experiments were carried out at pH 3.0 in the HAc-NaAc system.
3.2 Effect of contact time
The equilibrium adsorption time of Pr (III) on D72 resin was investigated. As shown in Fig. 2, a high initial slope for the adsorption curves is observed. It indicates that the initial uptake is rapid, since at the beginning of the adsorption process all the reaction sites are vacant and the extent of adsorption is high.After a rapid initial uptake, there is a transitional phase, in which the rate of uptake is slow with uptake reaching almost a constant value. Consequently, the adsorption is carried out in two distinct stages, a relatively rapid one followed by a slower one. The results also indicate that an increase of temperature enchances the capacity of praseodymium adsorption [14].
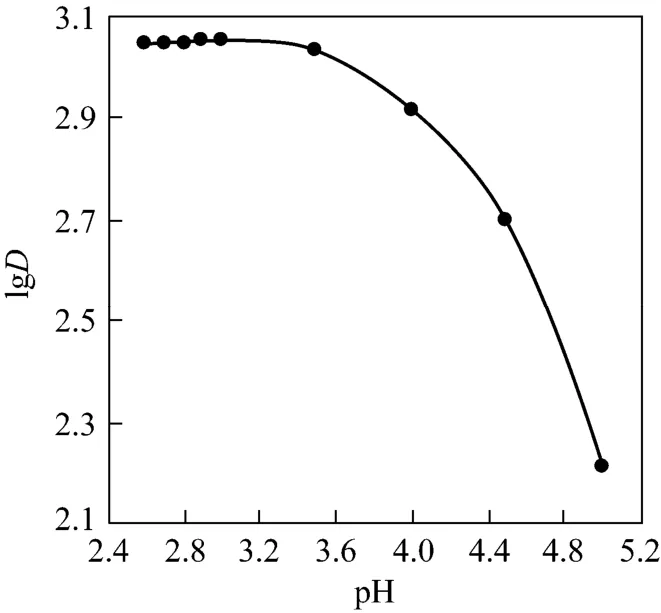
Figure 1 Effect of pH on the distribution coefficient (mass of resin=15.0 mg, C0=0.286 mg·ml-1, T=298 K, r=100 r·min-1)
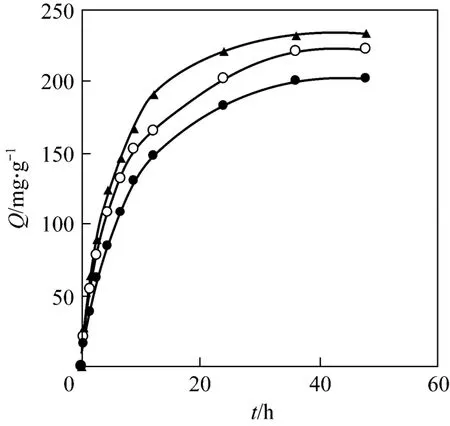
Figure 2 Effect of contact time for the adsorption (mass of resin=15.0 mg, C0=0.286 mg·ml-1, pH=3.00, r=100 r·min-1)● 288 K; ○ 298 K; ▲ 308 K
3.3 Adsorption kinetics study
The study of adsorption kinetics describes the solute uptake rate and evidently the rate controls the residence time of metal ion uptake at the solid-solution interface including the diffusion process. The mechanism of adsorption depends on the physical and chemical characteristics of the adsorbent as well as on the mass transfer process [15]. The experimental results were used to study the kinetics of metal ion adsorption. The kinetics of Pr (III) adsorption on D72 resin was analyzed using pseudo-first-order and pseudo-second-order models [16]. The conformity between experimental data and the model predicted values was expressed by correlation coefficient (R2).
The adsorption of Pr (III) from a liquid phase to solid phase can be considered as a reversible process with equilibrium between the solution and solid phase.For the pseudo-first-order model, the following relation was used for the variation of adsorbed concentration with respect to time [17].
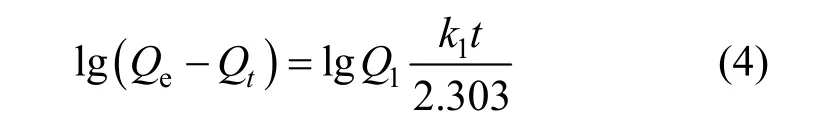
wherek1(h-1) is the rate constant of pseudo-first-order adsorption,Qe(mg·g-1) andQt(mg·g-1) denote the amounts of adsorption at equilibrium and at timet,respectively, andQ1(mg·g-1) is the calculated adsorption capacity. The slopes and intercepts of plots of lg(Qe-Qt)versustwere used to determine the pseudo first-order rate constantk1andQ1.
In addition, a pseudo-second-order equation based on sorption equilibrium capacity may be expressed in the form of [18]
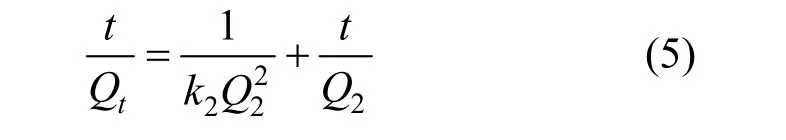
The constants (k2andQ2) can be experimentally determined by plottingt/Qtversustfrom the intercept and slope, respectively, and there is no need to know any parameter beforehand.
According to the parameters (Table 2), the experimental results obtained are found to obey the pseudo-second-order model. The theoreticalQ1values estimated from the pseudo-first-order model give significantly different values compared to experimental values, and the correlation coefficients are also found to be lower. These results show that the first-order kinetic model does not describe these adsorption systems. The theoreticalQ2values for the resin are very close to the experimentalQevalues in the case of second-order kinetics. The correlation coefficients for the pseudo-second-order equation are greater than 0.989.The pseudo-second-order equation at different temperatures fits well with the experimental data. The pseudo-second-order model is based on the assumption that the rate-determining step may be a chemical sorption involving valence forces through sharing or exchange of electrons between adsorbent and adsorbate [19]. Thus, successful fitting of this model suggests that chemisorption is the rate-controlling step.
From Table 2, it is seen that the adsorption capacity increases with temperature, showing an endothermic adsorption process. The activation energy is determined according to the pseudo-second order rate constant, expressed as a function of temperature by the Arrhenius equation [20].
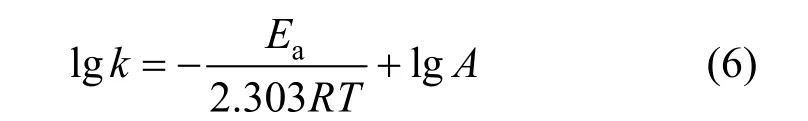
Value of the activation energy,Ea, can be determined from the slope of lgkversus1/T, and the value is 14.71 kJ·mol-1, which can be considered as a low energy barrier in this study. It can be deduced that the adsorption rate accelerates as temperature increases in the scope of experimental temperature.
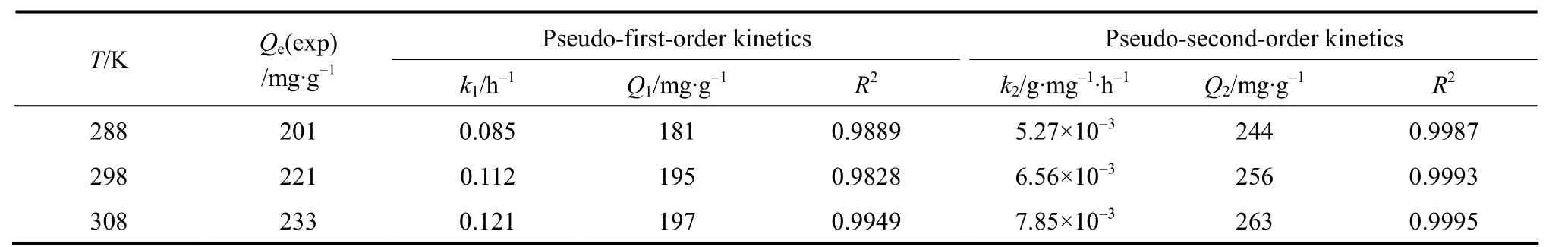
Table 2 The first-order and second-order kinetics parameters
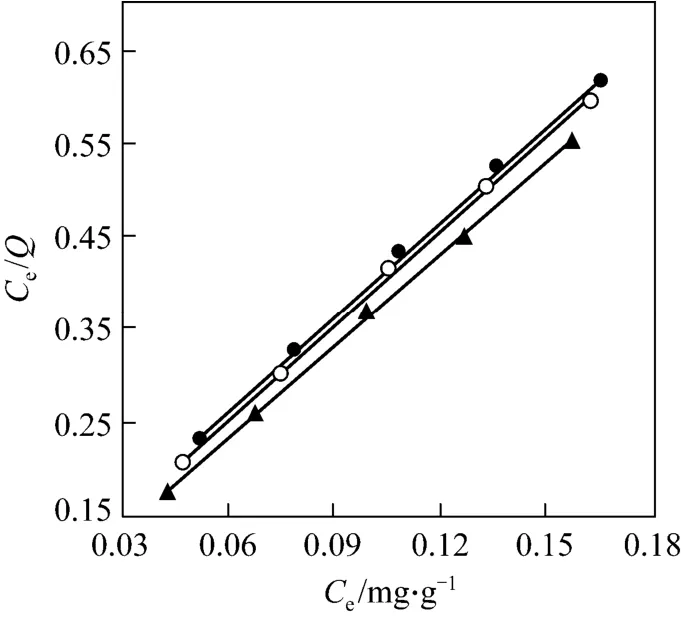
Figure 3 Langmuir isotherm (mass of resin=15.0 mg,C0=0.286 mg·ml-1, pH=3.00, r=100 r·min-1)● 288 K; ○ 298 K; ▲ 308 K
3.4 Equilibrium adsorption isotherms
Equilibrium data, commonly known as adsorption isotherms, are the basic requirements for the design of adsorption systems. Equilibrium data for a specific adsorbate/adsorbent system can be obtained experimentally, with a time-consuming procedure that is incompatible with the growing need for sorption system design. Analysis of equilibrium data is important for developing an equation that can be used to compare different sorbents under different operational conditions and to design and optimize an operating procedure [21, 22]. Langmuir [23, 24] and Freundlich[25] equations are used to reveal the linearity fitting and to describe how solutes interact with the resins.The linear forms of the Langmuir and Freundlich isotherms are represented as follows.
Langmuir isotherm:
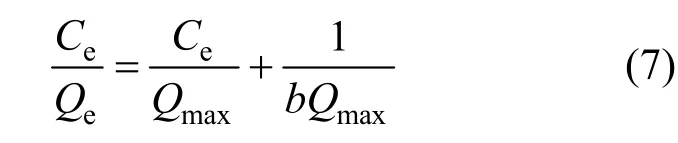
whereQeis the adsorption capacity in equilibrium state,Ceis the equilibrium Pr (III) concentration in solution,Qmaxis the maximum capacity of the adsorbent andbis the Langmuir constant which reflects quantitatively the affinity between the D72 resin and Pr (III).
Freundlich isotherm:
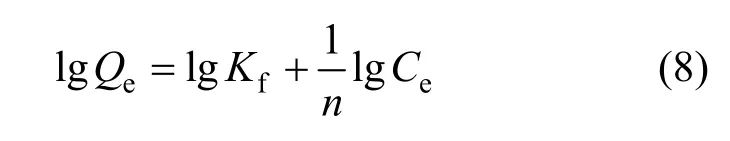
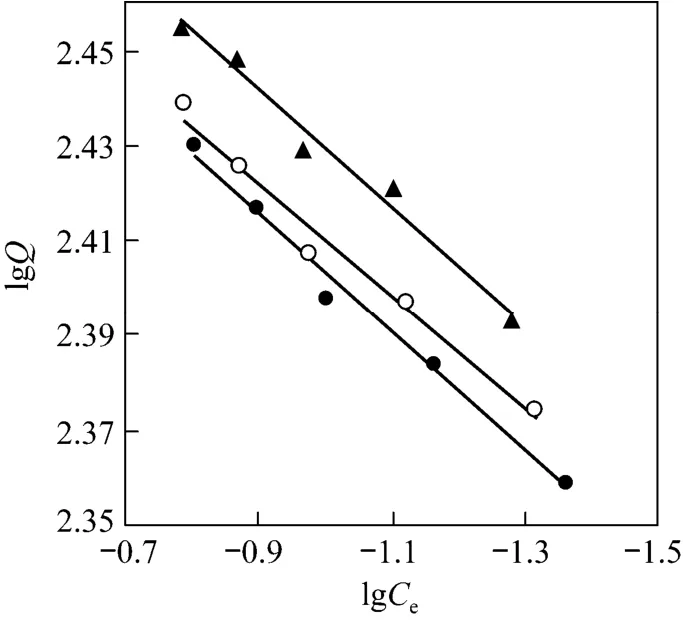
Figure 4 Freundlich isotherm (mass of resin=15.0 mg,C0=0.286 mg·ml-1, pH=3.00, r=100 r·min-1)● 288 K; ○ 298 K; ▲ 308 K
The Freundlich isotherm constantsKfandnare constants incorporating all factors affecting the adsorption process such as adsorption capacity and intensity of adsorption.
The Langmuir and Freundlich isotherms are shown in Figs. 3 and 4, and the parameters are listed in Table 3. It is evident that the adsorption of Pr (III)onto D72 resin fits the Langmuir isotherm model better than the Freundlich isotherm model, as indicated by theR2values and the adsorption capacity values in Table 3. Since the Langmuir isotherm assumes a monolayer coverage and uniform activity distribution on the adsorbent surface, this is an expected result.
The maximum adsorption capacity of the D72 for Pr (III) is evaluated to be 294 mg·g-1(2.09 mmol·g-1)for the Langmuir model at 298 K and the adsorption molar ratio (metal/functional group) of D72 resin for Pr (III) is 0.523. It means that the complex ratio of the functional group (SO3H) to Pr (III) is about 2∶1.
The essential features of a Langmuir isotherm can be expressed in terms of a dimensionless constant,separation factor or equilibrium parameterRL, which is used to predict that an adsorption system is “favorable” or “unfavorable” [26].

TheRLvalues for the adsorption on clarified sludge at initial concentration of 0.3 mg·ml-1are listed in Table 3. The results reveal that the adsorption of Pr(III) on D72 resin is a favorable adsorption.

Table 3 Langmuir and Freundlich parameters
3.5 Thermodynamic parameters
In order to evaluate the feasibility and the effect of temperature better, thermodynamic parameters such as standard free energy change (ΔGӨ), standard enthalpy change (ΔHӨ) and standard entropy change (ΔSӨ) are also evaluated [27]:

From the slope and intercept of the plot (Fig. 5),the values of ΔHӨand ΔSӨare computed, while ΔGӨis calculated using Eq. (11). The values of these parameters thus calculated are recorded in Table 4. It may be concluded from the positive values of ΔHӨthat the sorption process is endothermic while the positive value of ΔSӨis an indicative of increased randomness at the adsorbent-absorbate interface during the adsorption. The negative value of ΔGӨconfirms the feasibility and spontaneous nature of the adsorption process [28].
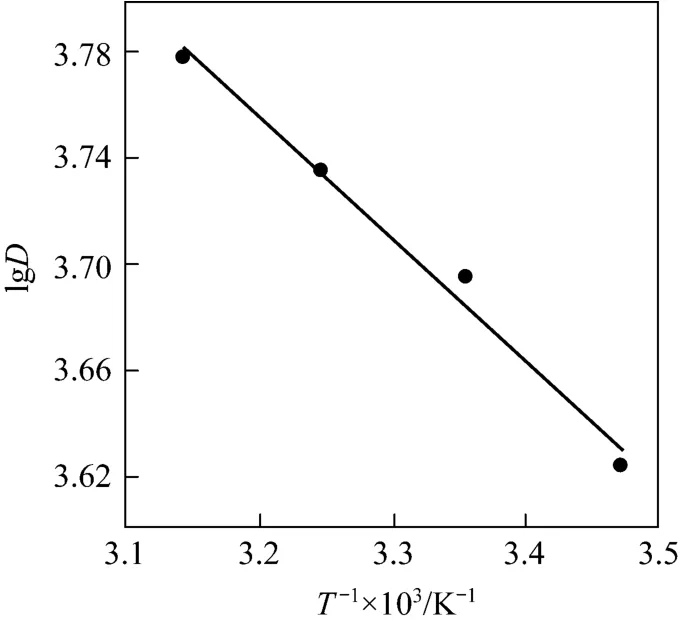
Figure 5 Relationship between lgD and T-1 (mass of resin=15.0 mg, C0=0.286 mg·ml-1, pH=3.00, r=100 r·min-1)
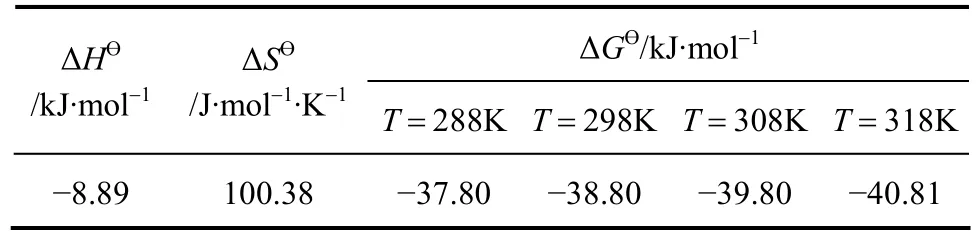
Table 4 Thermodynamic parameters for Pr (III) on D72 resin
3.6 Desorption studies
The economic feasibility of using an adsorbent to absorb metal ions from wastewater relies on its regeneration ability during multiple adsorption/desorption cycles. Adsorption of metal ions on any adsorbent can be by physical, chemical bonding, ion exchange or combination of all. Desorption study can give a clear idea about the mechanism of adsorption and is useful in recycling of the adsorbent and recovery of metals.In this work, desorption of Pr(III) with various concentrations of eluent solution are carried out. The results presented in Table 5 show that the elution ratio is different when the eluent concentration changes. Maximum recovery of Pr(III), at 100%, is achieved with the 1 mol·L-1HCl-0.5 mol·L-1NaCl eluent solution.The results show that the Pr (III) adsorbed by the D72 resin can easily be desorbed, which indicates that the resin can be employed repeatedly in Pr (III) adsorption.
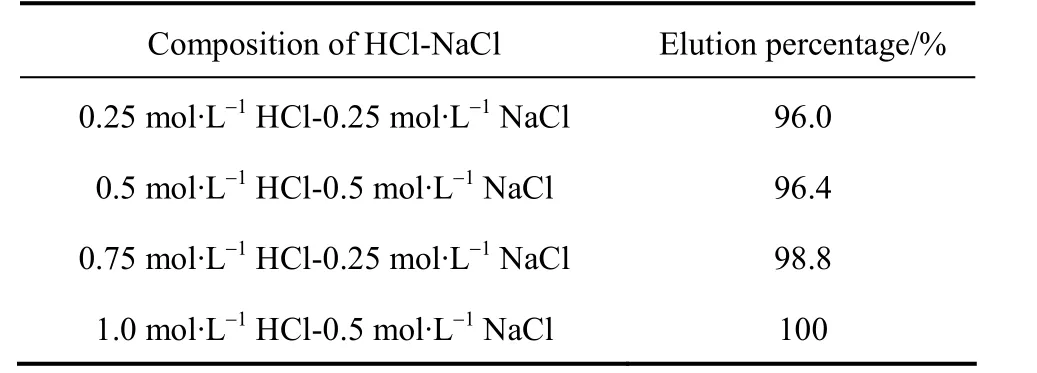
Table 5 The elution test of Pr (III)
3.7 Dynamic adsorption and desorption
3.7.1Dynamic adsorption curve
The performance of packed beds is described through the concept of the breakthrough curve. The breakthrough curve shows the loading behavior of Pr (III) to be removed from solution in a fixed bed and is usually expressed in terms of adsorbed Pr (III) concentration or normalized concentration defined as the ratio of effluent Pr (III) concentration to inlet Pr (III)concentration (Ce/C0) as a function of time or volume of effluent for a given bed height. The area under the breakthrough curve obtains by integrating the adsorbed concentrationversusthe throughput volume plot could be used to find the total adsorbed Pr (III)quantity (maximum column capacity). Total adsorbed Pr (III) quantity in the column for a given feed concentration and flow rate is calculated as follows [29]

The capacity valueQis obtained by graphical integration as 201 mg·g-1. Successful design of a column adsorption process requires prediction of the concentrationversustime profile or breakthrough curve for the effluent. The maximum sorption capacity of resin is also needed in design. Traditionally, the Thomas model is used to fulfill the purpose, which is [30]
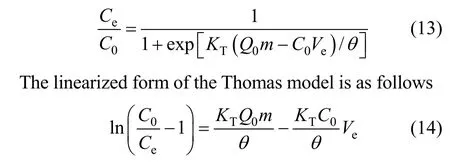
The kinetic coefficientKTand the adsorption capacity of the bedQ0can be determined from a plot of ln[(C0/Ce)-1]versustat a certain flow rate as shown in Fig. 6. The Thomas equation coefficients for Pr (III)adsorption areKT=1.42×10-2ml·min-1·mg-1andQ0=203 mg·g-1. The theoretical predictions based on the model parameters are compared with the experimental data as shown in Fig. 7.
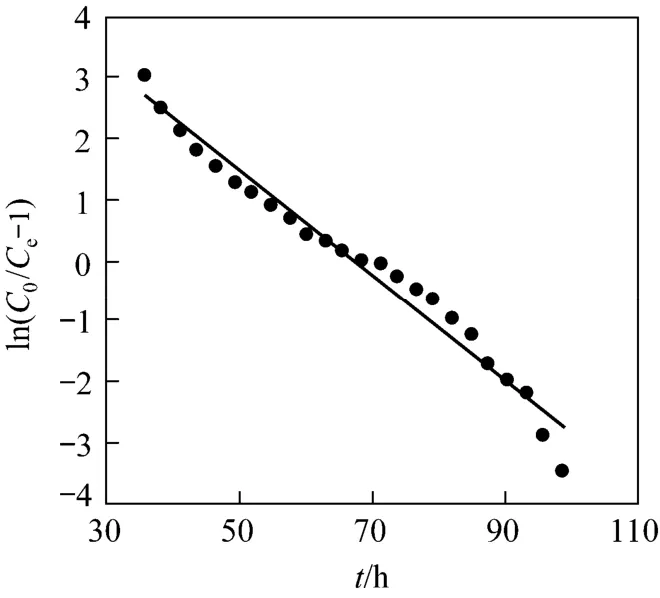
Figure 6 Linear plots of ln(C0/Ce-1) versus t by application of Thomas model (mass of resin=300 mg, pH=3.00,C0=0.10 mg·ml-1, θ =0.152 ml·min-1)

Figure 7 Dynamic adsorption curve (mass of resin=300 mg,pH=3.00, C0=0.10 mg·ml-1, θ =0.152 ml·min-1)● experimental data; △ Thomas model
The Thomas model is found in a relatively good fitness with breakthrough curves for adsorption of Pr (III) on D72 resin with a highR2value (0.9725),and the theoreticalQ0value is very close to the experimental one. Therefore, the experimental data fits well to the Thomas model.
3.7.2Dynamic desorption curve
Efficient elution of adsorbed solute from D72 resin in column is essential to ensure the reuse of resin for repeated adsorption/desorption cycles. With respect to the stripping of Pr (III) from D72 resin, 1.0 mol·L-1HCl-0.5 mol·L-1NaCl eluant is employed. Desorption curve is plotted with the effluent concentration (Ce)versuselution volume from the column at a certain flow rate. It can be seen from Fig. 8 that the adsorption flow rate is less so that the volume of elution is less, which helps in easy handling and high concentration for economical recovery of Pr (III). It is observed that the total volume of eluent is 150 ml, after which further desorption is negligible. Therefore, the 1.0 mol·L-1HCl-0.5 mol·L-1NaCl eluant is appropriate.
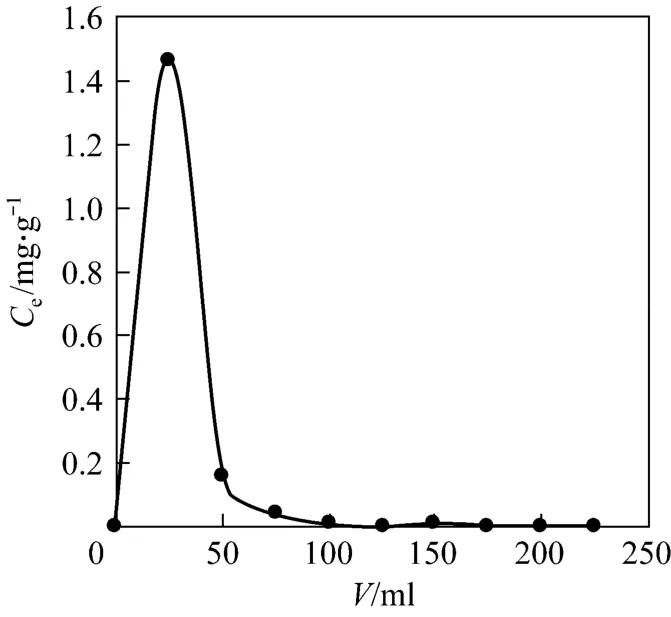
Figure 8 Dynamic desorption curve (mass of resin=300 mg, θ =0.10 ml·min-1)
3.8 IR spectra
From the results above, it can be deduced that the adsorption of Pr (III) by D72 resin is assigned to a chemical adsorption. Therefore, the functional group of D72 resin, SO3H, and Pr (III) are supposed to form chemical bonds. To identify this possibility, IR spectra are obtained for D72 resin before and after Pr (III) adsorption, as shown in Fig. 9. In general, significant changes are observed. The IR spectra of D72 resin exhibit major band at 3433 cm-1for OH stretching vibration. Medium broad vibrations observed in the IR spectrum in the range 1740-2786 cm-1are assigned to overtones/combinations of hydrogen bonded
OH bending modes from proton tunneling and Fermi resonance interactions [31-33]. The SO2asymmetric vibration is found to be at 1221 cm-1as a medium band in the IR spectrum [34]. The symmetric SO2stretching vibration appears around 1179 cm-1in both IR and Raman spectra. By comparison with SOH bending frequencies in sulfuric acid and other sulfonic acids, the band at ~1127 cm-1is assigned to SOH bend. A comparison of the spectra for free D72 resin with that of Pr loaded D72 resin reveals characteristic changes ofνOH,νasSO2andνsSO2, which shifts from 3433, 1221 and 1179 cm-1before Pr (III) adsorption to 3409, 1223 and 1156 cm-1Pr (III) after adsorption.These findings may suggest that there are coordination bonds between Pr (III) ion and oxygen atoms in the OH and S O groups in the adsorption.
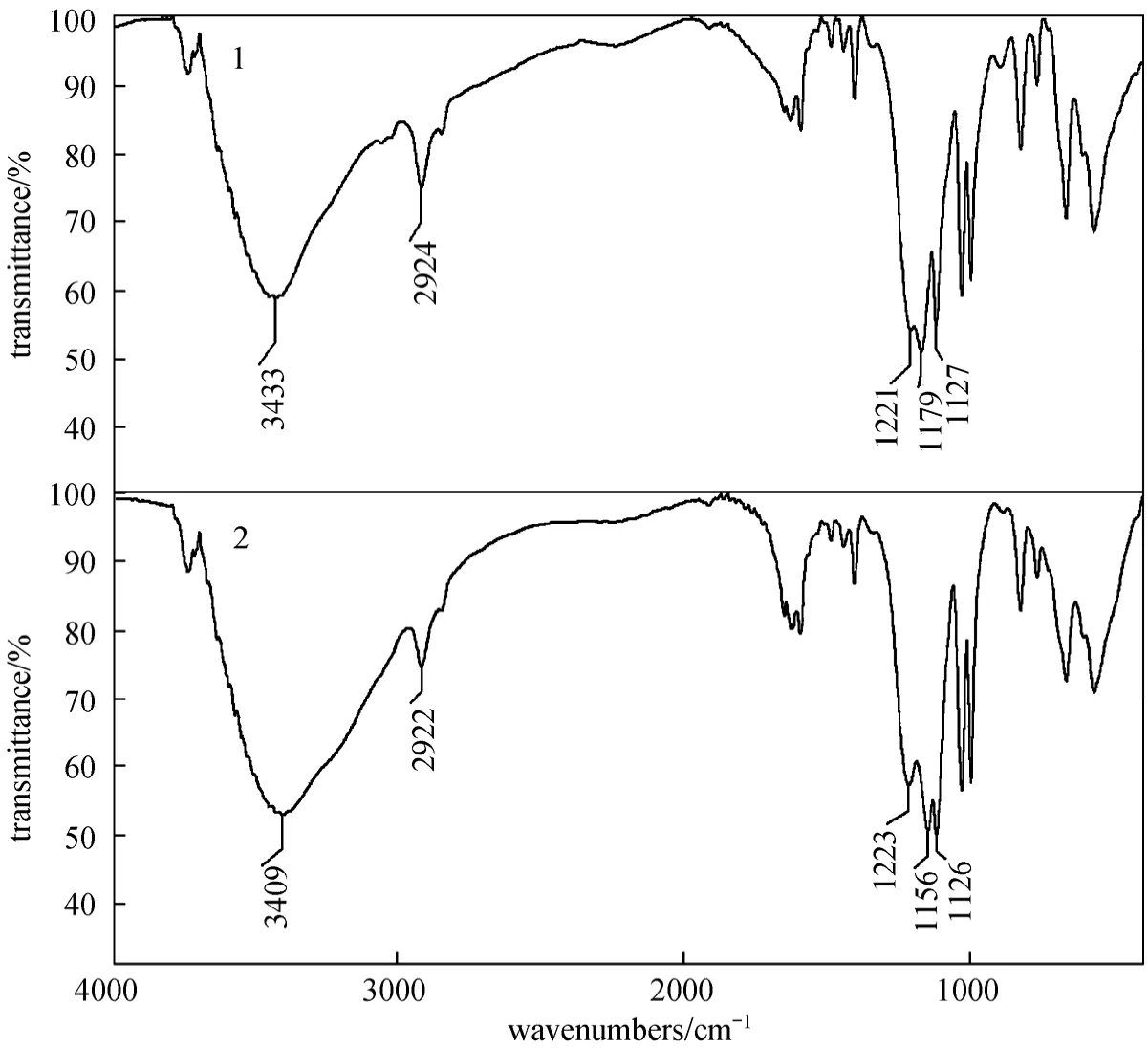
Figure 9 Infrared spectra1—before adsorption; 2—after Pr (III) adsorption
4 CONCLUSIONS
In this study, the adsorption characteristics are investigated at different pH values, temperatures and contact time by batch and column experiments. We conclude that D72 resin can be used for preconcentration of Pr (III) very effectively. It is observed that Pr (III) adsorption is highly dependent on pH. In addition,D72 resin dosage and initial Pr (III) concentration are effective on Pr (III) adsorption process. Kinetic studies show that the adsorption process obeys pseudo-secondorder kinetics, and the adsorption behavior can be modeled using the Langmuir isotherm. The maximum adsorption capacity of Pr (III) is estimated to be 294 mg·g-1by batch method at 298 K. The apparent activation energy is 14.71 kJ·mol-1. The adsorption parameters of thermodynamic are ΔHӨ=8.89 kJ·mol-1,ΔGӨ=-38.80 kJ·mol-1, ΔSӨ=100 J·mol-1·K-1, which indicates that the adsorption of Pr (III) on the D72 resin is endothermic in nature. Column experiments show that it is possible to adsorb Pr (III) from aqueous solutions dynamically. In summary, D72 resin have many advantages to adsorb Pr (III) in the solution and it has a potential for the treatment of industrial effluents containing rare earth elements.
NOMENCLATURE

1 El-Dessouky, S.I., El-Sofany, E.A., Daoud, J.A., “Studies on the sorption of praseodymium (III), holmium (III) and cobalt (II) from nitrate medium using TVEX-PHOR resin”,J.Hazard.Mater., 143(1-2), 17-23 (2007).
2 Hwang, D.W., Lee, J.S., Li, W., Oh, S.H., “Electronic band structure and photocatalytic activity of Ln2Ti2O7(Ln=La, Pr, Nd)”,J.Phys.Chem.B., 107 (21), 4963-4970 (2003).
3 Corradi, A.B., Bondioli, F., Ferrari, A.M., “Role of praseodymium on zirconia phases stabilization”,Chem.Mater., 13 (12), 4550-4554(2001).
4 Xu, H., Hu, X.L., Zhang, L.Z., “Generalized low-temperature synthesis of nanocrystalline rare-earth orthoferrites LnFeO3(Ln=La, Pr,Nd, Sm, Eu, Gd)”,Cryst.GrowthDes., 8 (7), 2061-2065 (2008).
5 Liang, P., Liu, Y., Guo, L., “Determination of trace rare earth elements by inductively coupled plasma atomic emission spectrometry after preconcentration with multiwalled carbon nanotubes”,Spectrochimica Acta Part B, 60 (1), 125-129 (2005).
6 Shi, Y.W., Tian, J., Hao, H., Xia, Z.D., Lei, Y.P., Guo, F., “Effects of small amount addition of rare earth Er on microstructure and property of SnAgCu solder”,J.Alloy Compd., 453 (1-2), 180-184 (2008).
7 Waqar, F., Jan, S., Mohammad, B., Hakim, M., Alam, S., Yawar, W.,“Preconcentration of rare earth elements in seawater with chelating resin having fluorinated β-diketone immobilized on styrene divinyl benzene for their determination by ICP-OES”,J.Chin.Chem.Soc.,56 (2), 335-340 (2009).
8 Schijf, J., Byrne, R.H., “Stability constants for mono- and dioxalato-complexes of Y and the REE, potentially important species in groundwaters and surface freshwaters”,Geochimica et Cosmochimica Acta, 65 (7), 1037-1046 (2001).
9 E
ofl-
CKsa+m
aansdhS,
rA2+. Mi
o.n,s“
E
frvo
aml
u
aaqt
iuoe
no
uosf szoelou
ltii
toen
A
s
ufsoinr
gth
bea tscohr pa
tni
vde
f i
rxeemd
o
bveadl column operations”,J.Hazard.Mater., 151 (2-3), 432-445 (2008).
10 El-Sofany, E.A., “Removal of lanthanum and gadolinium from nitrate medium using Aliquat-336 impregnated onto Amberlite XAD-4”,J.Hazard.Mater., 153 (3), 948-954 (2008).
11 El-Dessouky, S.I., El-Sofany, E.A., Daoud, J.A., “Studies on the sorption of praseodymium (III), holmium (III) and cobalt (II) from nitrate medium using TVEX-PHOR resin”,J.Hazard.Mater., 143(1-2), 17-23 ( 2007).
12 Ayoob, S., Gupta, A.K., “Insights into isotherm making in the sorptive removal of fluoride from drinking water”,J.Hazard.Mater.,152 (3), 976-985 (2008).
13 Yao, C.P., “Sorption behavior and mechanism of D113 resin for erbium”,J.Rare Earth., 25 (Suppl. 1.), 169-174 (2007).
14 Hafizi, M., Abolghasemi, H., Moradi, M., Alamdar Milani, S.,“Strontium adsorption from sulfuric acid solution by Dowex 50W-X resins”,Chin.J.Chem.Eng., 19 (2), 267-272 (2011).
15 Xiong, C.H., Yao, C.P., “Preparation and application of acrylic acid grafted polytetrafluoroethylene fiber as a weak acid cation exchanger for adsorption of Er(III)”,J.Hazard.Mater., 170 (2-3), 1125-1132(2009).
16 Hu, Q.H., Meng, Y.Y., Sun, T.X., Mahmood, Q., Wua, D.L., Zhua,J.H., Lu, G., “Kinetics and equilibrium adsorption studies of dimethylamine (DMA) onto ion-exchange resin”,J.Hazard.Mater.,185 (2-3), 677-681 (2011).
17 Yao, C.P., “Adsorption and desorption properties of D151 resin for Ce(III)”,J.RareEarth., 28 (Suppl. 1), 183-188 (2010).
18 Arica, M.Y., Bayramoglu, G., “Biosorption of reactive red-120 dye from aqueous solution by native and modified fungus biomass preparations ofLentinus sajorcaju”,J.Hazard.Mater., 149 (2),499-507 (2007).
19 Ho, Y.S., “Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods”,Water Res., 40 (1), 119-125 (2006).
20 Demirbas, A., Pehlivan, E., Gode, F., Altun, T., Arslan, G., “Adsorption of Cu(II), Zn(II), Ni(II), Pb(II), Cd(II) from aqueous solution on Amberlite IR-120 synthetic resin”,J.Colloid Interf.Sci., 282 (1),20-25 (2005).
21 Bayramoglu, G., Bektas, S., Arica, M.Y., “Removal of Cd(II), Hg(II)and Pb(II) ions from aqueous solution using p(HEMA/chitosan)membranes,J.Appl.Polym.Sci., 106 (1), 169-177 (2007).
22 Bayramoglu, G., Arica, M.Y., “Ethylenediamine grafted poly (glycidylmethacrylate-co-methylmethacrylate) adsorbent for removal of chromate anions”,Sep.Purif.Technol., 45 (3), 192-199 (2005).
23 Langmuir, I., “Adsorption of gases on plain surface of glass mica platinum”,J.Am.Chem.Soc., 40 (9), 1361-1403 (1918).
24 Huang, G.L.,Yang C., Zhang, K., Shi, J., “Adsorptive removal of copper ions from aqueous solution using cross-linked magnetic chitosan beads”,Chin.J.Chem.Eng., 17 (6), 960-966 (2009).
25 Freundlich, H., “über die adsorption in lösungen”,Z.Phys.Chem.,57, 385-470 (1906).
26 Aksu, Z., Tatli, A.I., Tunc, O., “A comparative adsorption/biosorption study of Acid Blue 161: Effect of temperature on equilibrium and kinetic parameters”,Chem.Eng.J., 142 (1), 23-39 (2008).
27 Lyubchik, S.I., Lyubchik, A.I., Galushko, O.L., Tikhonova, L.P., Vital, J., Fonseca, I.M., Lyubchik, S.B., “Kinetics and thermodynamics of the Cr(III) adsorption on the activated carbon from co-mingled wastes”,Colloids Surf.A:Physicochem.Eng.Aspects, 242 (1-3),151-158 (2004)
28 El-Kamash, A.M., El-Gammal, B., El-Sayed, A.A., “Preparation and evaluation of cerium(IV) tungstate powder as inorganic exchanger in sorption of cobalt and europium ions from aqueous solutions”,J.Hazard.Mater., 141 (3), 719-728 (2007).
29 Tabakci, M., Yilmaz, M., “Sorption characteristics of Cu(II) ions onto silica gel-immobilized calix[4]arene polymer in aqueous solutions:batch and column studies”,J.Hazard.Mater., 151 (2-3), 331-338(2008).
30 Shu, Z.N., Yang, M.H., “Adsorption of rhenium (VII) with anion exchange resin D318”,Chin.J.Chem.Eng., 18 (3), 372-376 (2010).31 Philip, D., Aruldhas, G., Journal of Raman Spectroscopy, 21 (3),155-214 (1990).
32 Rao, C.N.R., Chemical Applications of Infrared Spectroscopy, Academic Press, New York (1963).
33 Blinc, R., Hadzi, D., Hydrogen Bonding, Pergamon, New York (1957).
34 Colthup, N.B., Daly, L.H., Wiberly, S.E., Introduction to IR and Raman spectroscopy, Academic Press, New York (1975).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Reactive Distillation for Producing n-Butyl Acetate: Experiment and Simulation
- One Step Bioleaching of Sulphide Ore with Low Concentration of Arsenic by Aspergillus niger and Taguchi Orthogonal Array Optimization*
- Adsorption of Chlortetracycline from Water by Rectories*
- Optimization of Fermentation Media for Enhancing Nitrite-oxidizing Activity by Artificial Neural Network Coupling Genetic Algorithm*
- Effect of Propanoic Acid on Ethanol Fermentation by Saccharomyces cerevisiae in an Ethanol-Methane Coupled Fermentation Process*
- Numerical Study on Laminar Burning Velocity and Flame Stability of Premixed Methane/Ethylene/Air Flames*
