Lysosomal Exocytosis in Schwann Cells Contributes to Axon Remyelination*
2012-03-16GANGCHEN,ZHIJUNZHANG,ZHONGYAWEI等
INTRODUCTION
The myelin sheath is a multilayered membrane syste m,which provides electrical insulation around the axon and participates in bidirectional communication with neurons and the extracellular environment(Trapp et al.,2004).Myelin biogenesis is a complex process involving coordinated exocytosis,endocytosis,mRNA transport,and cytoskeletal dynamics.Abnormalities of myelin are common in lysosomal storage diseases(LSDs;Faust et al.,2010;Marsden and Levy,2010;Prolo et al.,2009),which are a group of~50genetically inherited disorders that are characterized by a deficiency of one or more specific lysosomal enzymes that causes an ac-cumulation of undigested material inside the lysosome(Ballabio and Gieselmann,2009;Walkley,2009).Most LSDs have a severe phenotype and frequently affect the brain,resulting in progressive cellular degeneration,including neuronal dysfunction or death,axonal loss,and demyelination.The myelin abnormality may be due to a combination of primary and secondary demyelinations.For example,primary demyelination is classically recognized as the predominant pathogenesis underlying white matter disease in the lysosomal disorders metachromatic leukodystrophy,multiple sulfatase deficiency,and globoid cell leukodystrophy,whereas Tay-Sachs disease,Farber’s disease,and mannosidosis display lesions of secondary demyelination due to neuronal dysfunction/death and axonal loss(Faust et al.,2010;Suzuki,2008;van der Knaap and Valk,2005).However,our understanding of the cellular role of lysosomes in the formation and maintenance of myelin re-mains limited.
The traditional view that lysosomes are simply end organelles in cellular degradative pathways has been challenged by recent studies,which emphasize their more essential role within the endosomal/lysosomal system (Blott and Griffiths,2002;de Duve,2005).Thus,the degradation and eventual recycling of complex molecules also regulate signal transduction pathways,both by receptor-ligand internalization and the generation of potent signaling molecules (Ballabio and Gieselmann,2009;de Duve,2005;Zhang et al,2007).Furthermore,a small number of cell types also use their lysosomes as regulated secretory organelles.These secretory lysosomes package additional secretory products,respond to extracellular stimuli,and fuse with the plasma membrane to release their contents(Blott and Griffiths,2002).
After injury to peripheral nerve,in the distal segment,one sees Schwann cell proliferation,myelin phagocytosis by both neuronal and non-neuronal cells,the formation of Bünger bands and axonal sprouting(Stoll et al.,2002).Axon sprouting occurs at a rate of 1~4mm per day and the initially unmyelinated fibers are soon invested by Schwann cells and become myelinated (Burnett and Zager,2004).The regrowth includes axon regeneration and remyelination,but the mechanisms of the expression and exocytosis of myelin protein in Schwann cells during this process are unclear.In this study,we found that the myelin protein P0,which is a 28-kDa glycoprotein that accounts for over half the total protein in compact peripheral nervous system myelin (Quarles,2002,2005),was stored in Schwann cell late endosomes/lysosomes.Further evidence indicated that Rab27a,a small GTPase required for secretory lysosome trafficking(Blott and Griffiths,2002),distributed in Schwann cell late endosomes/lysosomes and short hairpin-mediated knockdown of Rab27a prevented the Ca2+dependent lysosomal exocytosis in Schwann cells.Finally,we demonstrated that the remyelination of the regenerated sciatic nerve was reduced in Rab27a deficient ashen mice.
MATERIALS AND METHODS Materials
All chemicals were from Sigma(St Louis,MO)unless otherwise noted.PLP-GFP plasmid was kindly provided by Dr.M.Simons (Max-Planck-Institute for Experimental Medicine,Germany);CD63-GFP plasmid was kindly provided by Dr.G.Griffiths(Oxford University,Oxford,UK);the pSuper-siRNA Rab27asequence was 5’-GATC CCCAGACTCTGGAGTGGGGAAGTTCAAGAGACTTCCC CACTCCAGAGTCTTTTTTGGAAA-3’(top)and 5’-AGCT TTTCCAAAAAAGACTCTGGAGTGGGGAAGTCTCTTGA ACTTCCCCACTCCAGAGTCTGGG-3’(bottom).GFP-tagged Sh-RNA Rab27a constructs were generated by standard polymerase chain reaction techniques to introduce the desired restriction sites in the flanking sequences of the GFP and ShRNA Rab27aconstructs.Rab27aashmice were from Institute of Genetics and Developmental Biology,Chinese Academy of Sciences(for detail information,see:http://hpsd.csdb.cn/hpsd_3/data/ash.htm).
Cell Culture and Transfection
Animal protocols were reviewed and approved by the Animal Ethics Committee at the University of Nantong,China.Primary cultures of Schwann cells were prepared as described previously(Honkanen et al.,2007).In brief,Schwann cells were prepared from sciatic nerves of 1-to 3-day-old Sprague-Dawley rats.To inhibit the growth of fibroblasts,the culture medium was replaced with culture medium containing 10μMcytosine arabinofuranoside for 2days.Then,the medium was replaced with DMEM/10%FBS supplemented with 2 μMforskolin and 2ng/mL heregulin-β1.When the Schwann cell-enriched cultures reached confluence,complement-mediated immunocytolysis to eliminate residual fibroblasts was performed by incubating the culture in 4μg/mL of anti-Thy-1.1antibody for 2hon ice,and then with 400μL of rabbit complement for 1hat 37℃.After this treatment,>95%pure Schwann cells were obtained within 9days in culture,and the remaining non-Schwann cells were mostly fibroblasts as determined by immunohistochemistry.Schwann cells were transfected with plasmid DNA using the Lipo-2000technique 4or 5days after purification.
Fluorescent Imaging
Schwann cells were loaded with 250nMLysoTracker Red DND-99(Invitrogen-Molecular Probes,USA)in MEMwith 10%FBS for 10min at 37℃.The cells were then washed for 20min in extracellular solution(ECS)before transfer to the chamber for imaging under a confocal microscope with a 60× (numerical aperture 1.2;plan-Apochromat)water-immersion objective.The time courses of the changes in fluorescence of LysoTracker Red DND-99were obtained at an image interval of 5swithλemission>590nm andλexcitation=561 nm.A low laser power(less than 1%power)was used to avoid possible fluorescent bleaching.The decrease in fluorescence intensities of Lysotracker due to photobleaching was less than 5%over 10min.In some experiments,Schwann cells were transfected with EGFP-linked molecules and the fluorescence was detected at 505-525nm with excitation at 488nm.For combined Lysotracker dye and Ca2+imaging,Schwann cells were preloaded with 2μMFluo-4AM(Invitrogen-Molecular Probes,USA)for 30min before being loaded with Lysotracker.
In some experiments,cells were preloaded with 10μMmembrane-permeable Ca2+chelator BAPTA-AM(bis-(oaminophenoxy)ethane-N,N,N’,N’-tetra-acetic acid acetoxymethyl ester)for 45min at 37 ℃ Data analysis was performed with Metamorph software 5.0 (Universal Imaging,Downington,PA).
Immunostaining
Schwann cells growing on coverslips were fixed with 4%paraformaldehyde in PBS at 4℃for 10min,and permeabilized with 0.01%Triton X-100in PBS for 12min before being treated with 10%BSA for 1hat 25℃.Cultures were then stained with one or both of the following antibodies overnight at 4 ℃:mouse anti-P0and rabbit anti-cathepsin D(1∶500dilutions).After washing to remove excess primary antibodies,the cultures were incubated for 1hat room temperature with the fluorescence-conjugated secondary antibodies anti-mouse IgG-Cy3and anti-rabbit IgG-Alex-488,(Jackson Immunoresearch,West Grove,PA).Excess antibody was removed,and cells were imaged with the Lecia SP2confocal microscope.
Isolation of Lysosomes from Schwann Cells
Lysosomes from pure cultures of Schwann cells were isolated as described previously (Schütt et al.,2002;Zhang et al.,2007).In brief,cultured Schwann cells from 16flasks(growth area 75cm2,Corning)were suspended in 250mMsucrose/10mMTris/HCl buffer,pH =7.4(TS buffer)and disrupted by sonication on ice with two 15spulses 10sapart,at 20kHz and 90W (Sonics and Materials VCX 600 Watt,Danbury,CT).The disrupted cells were centrifuged at 1,300g for 15min to remove the nuclei and cell debris,and the supernatant was submitted to differential centrifugation at 26,000g for 25min to obtain a crude particulate fraction containing lysosomes,mitochondria,and endoplasmic reticulum.Lysosomes were separated from other organelles by isopycnic centrifugation on iso-osmotic Percoll (Amersham)gradients;25mL of 30%Percoll solution(7.5mL Percoll mixed with 17.5mL 250mMsucrose)was mixed with suspended crude particulates and centrifuged in a fixedangle rotor(Beckman 70Ti)at 60,000g for 90min.Two fractions were then separated,the upper and lower fractions corresponding to the major populations of mitochondria and lysosomes respectively,as confirmed by activity assay of the lysosomal markerβ-Hexosaminidase and the mitochondrial marker succinate dehydrogenase.
We stern Blotting and Enzymatic Activity Determination
Different subcellular fractions derived from centrifugation were diluted in triple the volume of TS buffer before centrifugation at 2 0,000g for 30min to remove Percoll and obtain the sediment of different fractions.Equal amounts of protein were mixed with sample buffer(Bio-Rad),boiled for 10min at 95 ℃,separated on an 8%SDS-polyacrylamide gel,and transferred onto nitrocellulose.The membrane was blocked for 1hin 5% (w/v)nonfat instant milk in TBS containing 0.05%Tween-20(TBST)and probed at 4℃overnight with antibodies to LAMP1(1∶500),β-1,4-Gal-T1(1∶300),cathepsin D (1∶500)or P0 (1∶500).After washing with TBST,the membrane was incubated for 1hwith horseradish peroxidase-conjugated secondary antibody (1∶3,000).Immunoreactivity was visualized using Super Signal West Pico Luminol/Enhancer Solution(Pierce)according to the manufacturer’s instructions.The#2fraction was treated with ECS,10μMionomycin,or 200μMGPN for 5min then the supernatants were collected to perform immunoblottings for P0.β-Hexosaminidase,succinate dehydrogenase,LDH,and alkaline phosphodiesterase activity assays were carried out as described previously (Schütt et al.,2002;Zhang et al.,2007).
Ce ll-surface Biotinylation
Schwann cells were treated with ECS or 10μMionomycin for 4min,and then cells were incubated with 0.5mg/mL sulfo-NHS-LC-biotin (Pierce)in phosphate-buffered saline(PBS)at 4℃for 30min,washed with cold PBS containing 100mMglycine,lysed with RIPA buffer.A volume of 150μL of total cell lysate fraction was incubated with equal volumes of streptavidin-sepharose (GE Healthcare)overnight,spun at 1 5,000g at 4℃for 15min(supernatant=intracellular fraction).Pelleted streptavidin beads were washed with RIPA buffer and biotinylated cell-surface proteins eluted by heating in 150μL Laemmli buffer at 100℃for 5min.Samples of cell fractions were subjected to SDS-PAGE followed by western blot.Calnexin antibody(Santa Cruz)at 1∶500dilution was used to detect calnexin as an intracellular control.
Su rgical Procedure
Adult male C3H (28.4±1.8g,n=8)and Rab27aashmice(27.9±2.2g,n=8)were used.For surgery,under sterile conditions the skin from the shaved lateral right thigh was scrubbed with antiseptic solution.Under deep anesthesia(intraperitoneal injection of sodium pentobarbiturate,30mg/kg body weight),the right sciatic nerve was exposed through a skin incision extending from the greater trochanter to the mid-thigh distally,followed by a muscle-splitting incision.After nerve mobilization,a transection was performed(neurotmesis)immediately above the terminal nerve ramification using straight microsurgical scissors.Immediate co-optation of the transected nerve stumps was performed with 8/0monofilament nylon epineurial sutures.The contralateral sciatic nerve was left intact in both groups and served as control.The animals were intensively examined for signs of autotomy and contracture during the postoperative period and none presented severe wounds,infections,or contractures.
Electron Microscopic Analysis
Nerve specimens of four randomly selected mice from each group were examined by electron microscopy.Electron microscopy experiments were performed as described(Wang et al,2005).In brief,a 2-mm segment of the sciatic nerve(right side)distal to the lesion site was removed,fixed,and prepared for quantitative morphometry of myelinated nerve fibers.A 2-mm segment of uninjured sciatic nerve(left side)was also removed as control.The groups of the nerve sections were blinded to the examiner.To assess fiber maturity,we calculated the g-ratio,the relation of axon area to total fiber area.The myelinated axon area was measured by digitally tracing the inner and outer layers of the myelinated fiber by ImageJ software (NIH Image; National Institutes of Health).The g-ratio data are displayed as a scatter plot against axon area.
Statistical Analysis
Data are presented as mean±s.e.m.Statistical comparisons were assessed with an analysis of variance or Student’s t-test;P<0.05was taken as significant.
RESULTS Myelin Protein P0Stored in Late Endosomes/Lysosomes in Schwann Cells
The composition of compact myelin membranes differs from that of other biological membranes,because of the presence of several major proteins that are relatively specific for myelin(Quarles,2005).Most prominent among these proteins are PLP and P0,which constitute half of the myelin protein content in the central and peripheral nervous system,respectively(Hudson,2004).Recent data indicated that PLP is internalized and stored in late endosomes/lysosomes in oligodendrocytes(Trajkovic et al.,2006).To examine whether late endosomes/lysosomes in Schwann cells can similarly accumulate PLP as in oligodendrocytes,wetransfected PLPEGFP plasmid into Schwann cells and found that PLP colocalized with the lysosome-tracker DND99 (a fluorescent dye that specifically labels lysosomes;Fig.1a).This result indicated that Schwann cell lysosomes may also be involved in myelin protein trafficking.
Next,we assessed P0expression in Schwann cells in vivo and in vitro.The process of myelination in peripheral nerves starts around birth and is completed in the next few weeks(Stewart et al.,1993;Woodhoo and Sommer,2008;Woodhoo et al.,2009).The sciatic nerves of Sprague-Dawley ratswere thus assessed at postnatal days P1,P14,and P60.We found that P0showed differential colocalization ratio with cathepsin D,a secretory lysosomal protease(Gardella et al.,2001),in the three developmental stages(0.905±0.053at P1,0.786±0.105at P14,and 0.542±0.173at P60;n=15 for each group),indicating its negative correlation with the maturation of myelin (Fig.1b,c).To further confirm the presence of P0in Schwann cell lysosomes,we used a wellcharacterized method to separate isolated enrichment of lysosomes(Zhang et al.,2007)from pure cultures of Schwann cells.The results showed that two fractions were separated,with the#1and#2fractions corresponding to the major populations of mitochondria and lysosomes respectively,as confirmed by activity assay of the lysosomal markerβ-hexosaminidase and the mitochondrial marker succinate dehydrogenase(Table 1).The markers for cytosol(lactate dehydrogenase),plasma membrane (alkaline phosphodiesterase),nuclei(DNA),and Golgi membrane (β-1,4-Gal-T1)were not detected in the#2fraction,indicating that it contained pure lysosomes(Table 1and Fig.1d).To detect whether lysosomes contained P0protein,we examined P0protein in the supernatant of the#1and#2fractions with 0.2%Triton or 200 μMglycylphenylalanine 2-naphthylamide(GPN),a substrate of the lysosomal exopeptidase cathepsin C that selectively induces lysosome osmodialysis(Jadot et al.,1984;Zhang et al.,2007),Western blot showed that lysosomal extracts contained abundant P0(Fig.1e).

Fig.1. Myelin protein P0stored in late endosomes/lysosomes in Schwann cells.(a)Example images showing the colocalization of transfected PLP-EGFP (green)with the lysosome-tracker DND99(red)in the cultured Schwann cell.(b)The sciatic nerves of Sprague-Dawley rats at P1,P14,and P60were immunostained for cathepsin D(green)and myelin protein P0(red).Right:overlay of the two fluorescence signals.Cath.D,cathepsin D.The boxed areas are shown at a higher magnification in the insets.Scale bars,10μm (3μm for enlarged images).(c)Summary of the colocalization ratio(Red/Green,Pearson’s correlation coefficient)of the two fluorescence signals(P0/cathepsin D)shown in a.Data analysis was performed with Metamorph software 5.0 (Universal Imaging Corp.,Downington,PA).The experiment was repeated three times.Error bars indicate s.e.m.(d)Example of a Western blot with anti-LAMP1,anticathepsin D,anti-β-1,4-Gal-T1antibodies from subcellular mitochondrial(fraction 1)and lysosomal(fraction 2)compartments,respectively,which were treated for 5min with 0.2%Triton after separated from the pure cultured Schwann cells by centrifugation and Percoll gradients.Similar blots were obtained from three experiments.(e)Example of a Western blot with anti-P0antibody from supernatant of#1fraction and#2fraction treated for 5min with 0.2%Triton(T)or 200μMGPN (G).Similar blots were obtained from three experiments.
Ionomycin Induces Lysosome Exocytosis in Schwann Cells
To label lysosomes,cultured Schwann cells were incubated with lysotracker DND99.After 5min,fluorescent puncta were colocalized with CD63-GFP puncta,indicating that they were lysosomes(Fig.2a).To test if an increase in[Ca2+]i(intracellular calcium concentration)stimulated the exocytosis of lysosomes,the calcium ionophore ionomycin was added to Schwann cells in a Ca2+-containing buffer(1mMCaCl2).The fluorescence intensity of puncta in Schwann cells was reduced by about 60%after 200sexposure to 10μMionomycin(Fig.2b).No apparent destaining occurred during the same period in control cells(ECS treated).
Lysosomal exocytosis was further examined by electron microscopy.In control Schwann cells,apopulation of vesicles with the characteristic morphology of lysosomes (Holtzman1989;Rodriguez et al.,1997)was observed throughout the cell body,(Fig.2c).In contrast,cells fixed after 4min treatment with ionomycin showed apparent decreased lysosomes(Fig.2d,h)that distributed mainly in the proximity of the plasma membrane(Fig.2e-g),consistent with the increased lysosomal exocytosis.
Additional evidence for Ca2+-dependent lysosomal fusion with the plasma membrane was provided by exposure of the luminal domain of the lysosome membrane protein LAMP1 on the cell surface.Surface staining with LAMP1indicates the fusion of lysosomal membranes with the plasmalemma(Reddy et al.,2001;Zhang et al.,2007).Immunostaining of Schwann cells with anti-LAMP1that recognizes the luminal N-terminal domain of LAMP1before permeabilizing the cells with methanol showed the surface presence of LAMP1,whereas second immunostaining of the cells after permeabilization revealed the cytoplasmic and surface LAMP1.Significantly increased surface staining occurred in ionomycin-treated but not control cultures(Fig.3a,b).A cell-surface biotinylation experiment was also used to detect the surface LAMP1protein in cultured Schwann cells.Biotin-labeled cell-surface proteins were captured by streptavidin coated sepharose beads.Western blot analysis was used to detect the presence of LAMP1and the intracellular control protein(calnexin).As shown in Fig.3c,Lamp1was found in both cell-surface and intracellular fraction,whereas calnexin was found only in the intracellular fraction.The increased surface LAMP1and decreased intracellular LAMP1in the ionomycin-treated cells indicated that ionomycin induced lysosomal fusion with the plasma membrane.
To direct examine the released lysosomal content into extracellular solution,cells were incubated at 37℃ with 10μMionomycin,and release of the lysosomal enzymeβ-Hexosaminidase was measured in the incubation buffer.This enzyme is frequently used as a marker for lysosomes,because 90%of the totalβ-Hexosaminidase is located in this compartment(Griffiths et al.,1990).A continuous release ofβ-Hexosaminidase,typically reaching 9%of the total enzyme content of the cells,was specifically induced by 4min treatment with ionomycin(Fig.3d).To test whether cell integrity was affected by the exposure to ionomycin,activity of the cytoplasmic enzyme lactate dehydrogenase(LDH)was measured in the incubation buffer of treated and untreated cells.The LDH levels in the incubation buffer after 4min were low in both conditions(Fig.4e),indicating that the observed release ofβ-Hexosaminidase induced by the ionophore was not due to cell lysis.
Regulated Exocytosis of Lysosomes in Schwann Cells
The finding that elevation of cytoplasmic Ca2+-induced lysosome exocytosis in Schwann cells prompted us to further examine directly regulated exocytosis of lysosomes by moni-toring the destaining of lysotracker-labeled vesicles.Neuronglia interactions can regulate Schwann cell secretion and protein expression,and glutamate is a well-known signaling molecule released from nerve terminals (de Groot et al.,2000).We found that extracellular application of glutamate(1mM)induced a gradual reduction in the fluorescence intensity of individual granules labeled with lysotracker to a plateau level of 74.1%of control after 200streatment(Fig.4a).

TABLE1.The markers for cytosol(lactate dehydrogenase),plasma membrane (alkaline phosphodiesterase),and nuclei(DNA)
After the sciatic nerve is transected,the local conditions are hypoxic and ischemic(Skovgaard et al.,2009).To determine whether lysosomes undergo exocytosis under such pathological conditions,we treated Schwann cell cultures with potassium cyanide(KCN),an inhibitor of oxidative phosphorylation that has been used in culture models to induce chemical hypoxia(Dubinsky and Rothman,1991;Zhang et al.,2007).We found that this is chemic insult induced about 50%destaining of lysotracker-labeled granules within 200sKCN treatment(Fig.3b),much greater than that induced by glutamate.
Both glutamate and KCN treatments induced elevation of[Ca2+]i,as revealed by fluorescence Ca2+imaging using Fluo-4(Fig.4a-c),suggesting that lysosome exocytosis induced by these treatments is Ca2+-dependent.To test this notion,we loaded Schwann cell cultures with the membranepermeable Ca2+buffer BAPTA AM,which maintains the[Ca2+]i at a low level,and assessed the glutamate-or KCN-induced destaining of lysotracker-labeled granules in Ca2+-free solution.We found that under this condition glutamateand KCN-induced destaining was prevented,indicating that[Ca2+]i elevation is critical for lysosome exocytosis.Furthermore,[Ca2+]i elevation was sufficient to induce lysosome exocytosis,because treatment of the cultures with the Ca2+ionophore ionomycin to directly increase[Ca2+]i induced significant destaining of lysotracker granules.This ionomycininduced destaining was greater than that induced by glutamate,presumably due to the higher and more persistent[Ca2+]i elevation(Fig.4c).Thus,Ca2+is both necessary and sufficient for lysosome exocytosis.
We also assessed the activity ofβ-Hexosaminidase released from Schwann cells by such physiological and pathological stimuli.Continuous release ofβ-Hexosaminidase specifically triggered by glutamate typically reached 1.25%and by KCN 8.2%of the total enzyme content of the cells after 4min treatment(Fig.4d).During the same period,the activity of cytoplasmic LDH was measured in the incubation buffer of treated and untreated cells.LDH levels after 4min were low in both conditions(Fig.4e),indicating that the induced release ofβ-Hexosaminidase was not due to cell lysis.
Rab27aPlays a Central Role in the Exocytosis of Lysosomes in Schwann Cells
The small GTPase Rab27ahas been implicated as a marker for secretory lysosomes(Blott and Griffiths,2002),and sev-eral reports have highlighted its role in exocytosis(Munafóet al.,2007;Wilson et al.,2000).In particular,Rab27aplays a fundamental role in the exocytic mechanism of secretory lysosomes or lysosome-related organelles including melanosomes,lytic granules,platelet-dense granules,and basophil granules(Munafóet al.,2007).The subcellular distribution of endogenous Rab27awas examined by immunofluorescence and confocal microscopy.We detected the presence of Rab27ain Schwann cells and found it was colocalized with LAMP1(Fig.5a).To elucidate the function of Rab27ain Schwann cells,we first optimized the downregulation of Rab27aexpression using siRNA.Schwann cells transfected with Rab27ashRNA plasmid showed a dramatic decrease(85%)in the expression level of Rab27a,while control shRNA had no effect(Fig.5b).We found that the destaining of the lysotracker-labeled puncta induced by ionomycin was significantly decreased in Rab27a-downregulated cells compared with cells transfected with nonsilencing control shRNA (Fig.5c,d).Of note,the destaining of lysotracker-labeled puncta was markedly reduced but not abolished in Rab27a-downregulated cells.This is most likely explained by the fact that residual Rab27ais present in shRNA-treated cells.Altogether,these results suggest a central role for Rab27ain the secretory function of Schwann cells and in particular,in the release of the contents of lysosomes.
Rab27aash Mice Exhibit Deficits in Sciatic Nerve Regeneration and Remyelination
The physiological importance of the Rab27aprotein in granule exocytosis is evident,because mutations in the Rab27agene cause defects in granule exocytosis in cytotoxic T lymphocytes in human hemophagocytic syndrome(Griscelli syndrome;Ménaschéet al.,2000)and ashen mice(Wilson et al.,2000).As Schwann cell lysosomes contain myelin protein P0and Rab27aplays a central role in the process of regulated lysosome exocytosis,we hypothesized that Rab27a plays a central role in pe-ripheral nerve regeneration and remyelination.To test this hypothesis,we performed an endto-end anastomosis after sciatic nerve transection in Rab27aashand wild-type mice.The degree of regeneration and remyelination were determined by electron microscopic evaluation and electrophysiological measurements.
Ultrathin sections of specimens from the sciatic nerve tissues at the distal stump were analyzed by electron microscopy 4weeks after transection.There were gross morphological differences in the remyelination of the regenerated axons in the two groups(Fig.6a,b).In the wild-type mice,myelinated fibers had a compact and uniform structure,including a clear,electron-dense myelin sheath and perfect basal membrane of Schwann cells,in sharp contrast to that in the Rab27aashmice(Fig.6a-h).The g-ratio(the ratio of the axon area with whole fiber area)in the Rab27aashmice was significantly larger than that in the wild-type mice(P <0.01;Fig.6c).The average number of myelin layers per axon in the Rab27aashmice(21.31±1.49,n=100,four mice)was significantly fewer than that in the wild-type mice(31.73±1.53,n=100,4mice;Fig.6d-h,P<0.01).Thus,the regenerated myelinated fibers in the Rab27aashmice were significantly smaller in diameter and thinner in myelination than that in the wild-type mice.We also measured the contralateral normal nerves in both groups∶ the g-ratio was 0.51±0.021(n=100,four mice)in the wild-type and 0.52±0.035(n=100,four mice)in the Rab27aashmice;the mean number of layers was 53.42±1.23(n =100,four mice)in the wildtype and 51.23±1.31 (n=100,4mice)in the Rab27aashmice.There were no significant differences in the uninjured nerves between the two groups.In summary,the development of sciatic nerve appears to be unaffected by the rab27a mutation.In electrophysiological evaluation,compound muscle action potentials(CMAPs)are indicators of recovery of motor nerve function.Four weeks after transection,the distal and proximal CMAPs were measured on both the operated and unoperated sides in Rab27aashand wild-type mice (Fig.6i-k).The mean amplitudes of the CMAPs in the transected sciatic nerve in the Rab27aashmice(n=8)were 1.82±0.13mV (distal)and 1.70±0.16mV (proximal),respectively,significantly smaller than that in the wild-type mice(n=8),which were 3.92±0.25mV (distal)and 3.56±0.33mV (proximal),respectively.In the unoperated sciatic nerve,the mean CMAP was 7.24±0.34mV in the wild-type mice and 7.12±0.25mV in the Rab27aashmice(not statistically different).The recovery index of CMAP amplitude is calculated by the formula:Recovery index= Peak amplitude of the operated side/Peak amplitude of the unoperated side(Suzuki et al.,2000).The values were 54.14% (distal)and 49.17% (proximal)in wild-type mice,and 25.56% (distal)and 23.88% (proximal)in Rab27aashmice,showing that the Rab27aashmice had impaired regeneration and remyelination after nerve transection.

Fig.2. Ionomycin induces lysosome exocytosis in cultured Schwann cells.(a)Example images showing that Lysotracker DND99-labeled puncta (red)colocalized with the lysosomal membrane marker GFP-CD63(green)transfected in a Schwann cell.(b)Destaining of the DND99dye-labeled puncta in Schwann cells by ionomycin.Left and middle panels:example images of DND99-labeled puncta before and after perfusion with ionomycin for 200s.Right panel:time course of ionomycin-induced destaining,normalized to that obtained before ionomycin application(marked by the arrow).No destaining occurred during the same period in control cells(ECS treated).Error bars indicate s.e.m.(c-g)Transmission electron micrographs of cultured Schwann cells.(c)A population of vesicles with the characteristic morphology of lysosomes distributed throughout the cell body in ECS-treated cells.(d)Few lysosomes were detected in the ionomycin-treated cells.(e-g)Example samples showing lysosomes distributed at the proximity of plasma membrane in ionomycin-treated cells.Scale bars,10μm for a-c,2μm for e,f and 0.5μm for g-i.(h)Summary data showing the average number of lysosomes per cell in control-and ionomycin-treated cultures as shown in e and f.Error bars represent s.e.m.(“*”P< 0.01,as compared with the control group,Student’s t-test).The number associated with each column refers to the number of cells examined for each condition.
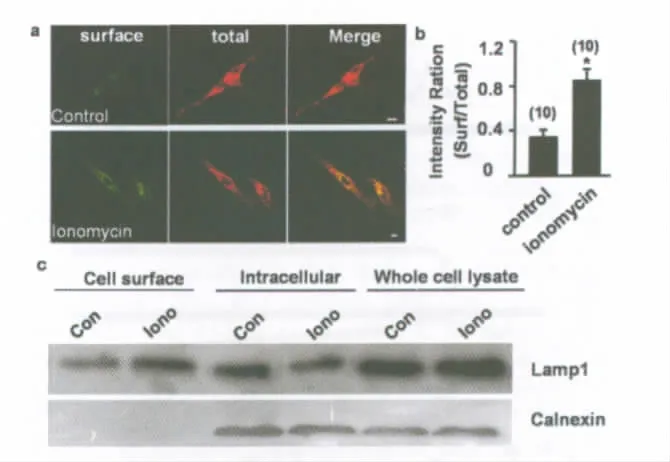
Fig.3. Ca2+-dependent lysosomal fusion with the plasma membrane.(a)Immunostaining of Schwann cells with anti-LAMP1that recognizes the luminal N-terminal domain of LAMP1before permeabilizing the cells with methanol shows the surface presence of LAMP1(surface,green),whereas the second immunostaining of the cells after permeabilization reveals the cytoplasmic and surface LAMP1(total,red).Note significantly increased surface staining in ionomycin-treated(lower panels),as compared with control(ECS-treated,upper panels)cultures.(b)Summary data showing the ratio of surface/total anti-LAMP1staining in control-and ionomycin-treated cultures as shown in(a).Error bars represent s.e.m.(“*”P<0.01,as compared with the control group,Student’s t-test).The number associated with each column refers to the number of cells examined for each condition in different three experiments.(c)Cellsurface Lamp1protein was detected by biotinylation.Western blot analysis was used to detect the presence of Lamp1,or the intracellular control protein (calnexin).Similar blots were obtained from three experiments.
DISCUSSION
The glycoprotein P0,the most abundant protein of peripheral nerve myelin,is believed to be involved in the compaction of the myelin sheath (Eichberg,2002).P0gene expression occurs constitutively long before myelination in the neural crest,as well as in embryonic nerves and Schwann cell precursors,and is independent of axons(Lee et al.,1997).It is generally believed that Schwann cells do not synthesize P0,unless they are induced to do so by continual positive signaling from axons.In contrast,Cheng and Mudge(1996)reported that cultured Schwann cells make large amounts of P0 without any axon-like signal,provided they have not been exposed to serum during the culture process.In this study,we also did not detect expression of P0in cultured Schwann cells by immunochemistry.However,when we used forskolin to stimulate Schwann cell proliferation and collected the purified lysosomes,we found that the lysosomes contained abundant P0.It has been suggested that cAMP may mediate part of the axon-to-Schwann cell signaling process to trigger myelination in vivo (Jessen and Mirsky,1992;Jessen et al.,1991).It is thus possible that agents such as forskolin that elevate intracellular cAMP levels can induce the expression of P0in neuron-free Schwann cell cultures(Mews and Meyer,1993;Morgan et al.,1994).
It is recognized that P0is synthesized in the endoplasmic reticulum,sorted into specific vesicles on exit from the trans-Golgi network and targeted to the Schwann cell plasma membrane,eventually to become the major constituent of compact myelin(Trapp et al.,1995;Voshol et al.,1996).Newly synthesized P0appears in myelin only about 30min after its synthesis in the endoplasmic reticulum.In the absence of myelin assembly,the Schwann cells therefore dramatically alter the biosynthetic rate and post-translational processing of this myelin component (Rapaport and Benjamins,1981;Pareek et al.,1997).By performing precursor pulse-chase analyses to examine the metabolic fate of P0in the transected nerve,it was suggested that Schwann cells of the transected nerve regulate the intracellular levels of P0by degrading the glycoprotein by specific delivery to the lysosome 1~2hafter biosynthesis(Brunden and Poduslo,1987).However,in this study,we found that lysosomes in Schwann cells may actively transport P0protein to the plasma membrane and contribute to the remyelination after sciatic nerve regeneration.
The view that the lysosome is simply a terminal degradation compartment has recently been challenged by the finding that some cells store their secretory proteins in lysosomes,which are functionally unusual in that they serve both as a degradative and as a secretory compartment and are thus defined as secretory lysosomes.Here,we demonstrated that elevation in the intracellular free Ca2+concentration of Schwann cells induced fusion of lysosomes with the plasma membrane.This was verified by the decreased fluid-phase trackers previously loaded into lysosomes,the reduced number of lysosomes in the cytoplasm,the increased lysosomal enzymeβ-Hexosaminidase in extracellular medium,and the appearance of the lysosomal protein LAMP1on the plasma membrane.We also found that Schwann cell lysosomes exhibited different degrees of exocytosis,depending on the nature of the stimulus,implicating physiological effects of neuroligands(glutamate)and pathological effects of ischemic insult(KCN)on lysosome exocytosis.

Fig.4. Ca2+-dependence of lysosome exocytosis in Schwann cells.(a,b)Changes in the fluorescence signals of lysotracker DND99-labeled puncta and Ca2+ dye Fluo-4AMinduced by perfusion with glutamate(a)and KCN(b),with or without preloading of BAPTAAM.Data are normalized to the average fluorescence intensity measured during 50s(control period)before the onset of drug perfusion(marked by the arrow).(c)Left:Percentage destaining of lysotracker DND99after perfusion with ionomycin,KCN or glutamate for 150~200sunder the conditions shown in a and b.The number above each column refers to the number of cells examined.Error bars indicate s.e.m.Right:Average changes in the intensity of Fluo-4AMfluorescence obtained during perfusion with ionomycin,KCN or glutamate between 0and 50s(peak phase;open columns)or 150and 200s(sustained phase;filled columns),normalized to the Fluo-4signal obtained during the 50sbefore perfusion for each cell,under the conditions shown in a and b.The number above each column refers to the total number of cells examined.Error bars indicate s.e.m.(d)Time course of the release ofβ-Hexosaminidase(hexosam,left)and lactate dehydrogenase(LDH,right)from control cultures(white columns),and cultures treated with 1mMglutamate(grey columns),4mMKCN(black columns)or 10μMionomycin(striped columns).Enzyme activity was normalized to the value obtained for the control group.Data represent averaged results from at least six experiments for each group;error bars indicate s.e.m.

Fig.5. Rab27aplays a central role in the exocytosis of lysosomes in Schwann cells.(a)Immunostaining with Rab27a(red)and LAMP1(green)was colocalized in Schwann cells.Scale bars,10μm.(b)Western blot showing a dramatic decrease in the Rab27aexpression in cultured Schwann cells transfected with EGFP-shRNARab27aas compared with the scramble control.(c)Images of puncta labeled by the lysotracker DND99(red)before(upper panels)and after(lower panels)exposed to 10μMionomycin for 200sec.Note that the destaining of lysotracker DND99-labeled puncta was significantly decreased in EGFP-shRNA-Rab27a-transfected cells(green).Right:overlay of the two fluorescence signals.Scale bars,10μm.(d)Average fluorescence intensity of the puncta labeled by lysotracker DND99after treatment with ionomycin,as shown in c.Data were normalized to the average fluorescence intensity of the puncta measured before ionomycin treatment in the same field(control).Error bars indicate s.e.m.
Early studies of freshly dissociated cell suspensions and frozen sections from rat sciatic nerve indicated that among the myelin lipids,the glycolipids galactocerebroside and sulfatide are present on the surface of many Schwann cells at least 1 day before myelination starts(Mirsky et al.,1980;Winter et al.,1982).These experiments also established that Schwann cells require contact with an appropriate axon to maintain high levels of myelin components,because these cells lose the glycolipids and myelin proteins over the first few days in culture.During the first postnatal week,clathrin-coated pits are prominently associated with rat myelin membranes,possibly indicative of an active exocytotic or endocytotic process during this maximal period of myelin assembly (Trapp et al.,1995).In this study,we found that P0has high colocalization ratio with the secretory lysosome marker cathepsin D in the newborn rat sciatic nerve,and the ratio decreases following myelination in adult animals.This phenomenon indicated that the secretory lysosomes in Schwann cells may be involved in the formation of myelin.The decreased colocalization ratio of P0/cathepsin D with the gradual maturation of myelin iscaused by the increased P0expression on the plasma membrane in adult Schwann cells.Our results are consistent with recent studies showing that a portion of the PLP is present in a LAMP1-positive late endosome/lysosome compartment in oligodendrocytes,and neuronal signals increase the transport and recruitment of late endosome/lysosome carriers to the plasma membrane(Simons and Trajkovic,2006;Trajkovic et al.,2006).
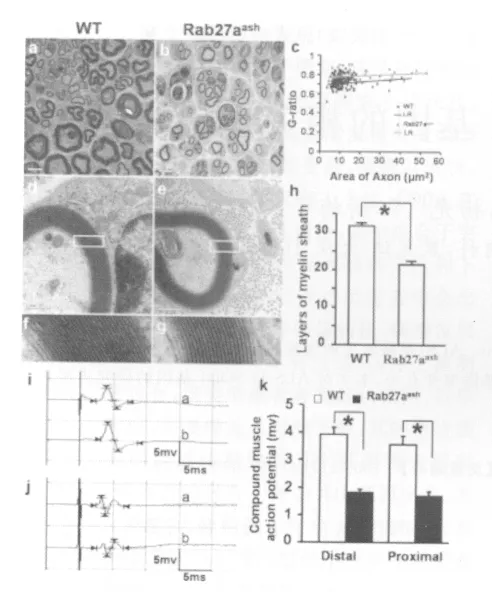
Fig.6. Rab27aash mice exhibit deficits in sciatic nerve regeneration and remyelination.(a-h)Ultrathin sections of specimens from the distal stump of the sciatic nerve were analyzed by electron microscopy 4weeks after nerve transection to compare numbers of regenerating unmyelinated and myelinated axons.Transmission electron micrographs of the regenerated nerves in wild-type (a,d,f)and Rab27aash mice(b,e,g).Scale bars,5μm for a,b and 0.5μm for d,e.(c)Scatter plot of g-ratio of myelinated fibers in the distal stump 4 weeks after nerve transection.Each spot represents the g-ratio of one myelinated fiber.The gratio,calculated by dividing the axon area(without myelin)by the total fiber area(including myelin),in the Rab27aash mice(0.78±0.005,n=100,four mice)was significantly larger than that in the wild-type mice(0.71±0.006,n=100,four mice)(“*”P <0.01,as compared with the wild type group,Student’s t test).Blue:wild-type mice;red:Rab27aash mice;LR:linear regression.n=100,four mice;(“*”P< 0.01,as compared with the wild type group,Student’s t test).(h)Statistical results of the mean number of myelin layers in wild type and Rab27aash mice as shown in d-g.Error bars represent s.e.m.(“*”P< 0.01,as compared with the wild type group,Student’s t test).n=100from four mice.(i-k)CMAP measurements at 4weeks after nerve transection.Representative traces of CMAP evoked by stimulating the distal(upper trace,a)and proximal(lower trace,b)ends of the transected nerve in a wild-type(i)and a Rab27aash(j)mouse.(k)Comparison of CMAP amplitudes in the two groups.Error bars represent s.e.m.“*”P< 0.01,as compared with the wild type group,Student’s ttest)n=8.
Rab27ais a tissue-specific Rab that associates with secretory lysosomes(Blott and Griffiths,2002)and intracellular protein trafficking (Chen et al.,1997).Mutations in the Rab27agene cause pigmentary dilution and immunodeficiency in human Griscelli syndrome(GS)type 2(Ménaschéet al.,2000)and a lightened coat color in ashen mice(Wilson et al.,2000).Neurological manifestations in patients with GS2 have been related to lymphocyte infiltration of the CNS(de Saint Basile and Fischer,2001;Ménaschéet al.,2000).However,neurological impairment has not been characterized in ashen mice.In this study,we found that the Rab27ais expressed in Schwann cell and is crucial for remyelination of axons in adults but not for myelination of axons in developmental stage.This result does not dismiss a role for Rab27a in myelination but suggests that Rab27amay be a redundant signal for myelination during developmental stage.It is noted that genes that regulate remyelination in adults are not always required for myelination during early development.For example,although Olig1in mice is not required for developmental myelination,it is indispensible for repairment of demyelinated lesion in adult CNS(Arnett et al.,2004)as well as for the formation of normal myelin in myelin-associated glycoprotein deficient mice(Li et al.,1994;Mukhopadhyay et al.,1994).
The hallmark of myelination is membrane biosynthesis,the synthesis and bulk transport of proteins to produce a unique extension of the glial cell plasma membrane that wraps around the axon.Although the exact underlying mechanisms regulating myelin biogenesis are unknown,our experiments support the concept that secretory lysosomes in Schwann cells play a key role in remyelination after sciatic nerve regeneration.This finding points to an interesting link between intracellular transport and myelin biogenesis.The challenge will now be to integrate the signaling and trafficking pathways toattain a comprehensive view of how myelination is regulated.
Acknowledgements
The authors thank Dr.I.C.Bruce for critical comments on the manuscrip t.
