Comparison and Analysis of Fabric Deodorization Test Methods
2012-02-07WEIMengyuan魏孟媛XUEWenliang薛文良LUWeimin陆维民MAJieCHENZhifei陈智菲
WEI Meng-yuan(魏孟媛),XUE Wen-liang(薛文良),LU Wei-min(陆维民),MA Jie(马 洁),CHEN Zhi-fei(陈智菲)
1 Shanghai Entry-Exit Inspection and Quarantine Bureau,Shanghai 200135,China
2 Engineering Research Center of Technical Textiles,Ministry of Education,Donghua University,Shanghai 201620,China
Introduction
There are a lot of microorganisms around people and they actfrequently in a certain temperature and humidity,decomposing the protein,carbohydrate,higher fatty acid,and something else from human body.While the odorof decomposition spreads in the air and goes into the nasal cavity of human body,it is adsorbed by smell cells of the upper nasal cavity causing membrane potential which is sent into the brain as pulse signal along the nerves.Therefore,people can feel the dirty smell[1].The dirty smell refers to the unpleasant odor for most people.And the root of the dirty smell is mostly from the decomposition of microorganisms.Faced with the dirty smell,we use variety of methods to eliminate stink for comfort[2-4]including developing antibiosis deodorization fabric[5,6],physical coating[7,8],and photo catalyst[9,10]and so on.So far,more and more deodorization fabric products enter the market as the demands of people.But it's hard for consumers to distinguish them.From the rights and interests of consumers and environment coordination,the deodorization test methods and relative standards should be studied and defined as soon as possible.So that it can make the deodorization product trade more standard and protect the legitimate rights and interests of consumers.
Although early researchers used differentmethodsto measure the deodorizing performance of the products as well as developing the deodorizing textile,these methods were not uniform,and the testing procedures were very rough.Therefore,there is lack of appropriate method to indict the deodorizing performance in the textile trade.In this paper,the detector tube method and gas chromatography-flame ionization detector(GC-FID)method were chosen to study the deodorizing rates of fabric and the influencing factors on deodorization test were discussed.On this basis,the method of testing the deodorizing performance for the textile was built.
1 Experimental
The odorous substance is divided into three categories in daily life:alkaline,acidity,and organic.So in this paper,the typical gases were used for testing by detector tube method and GC-FID method[11-13].Although GC-FID method is widely used in the qualitative and quantitative testing of organic substance,the parameter of GC-FID should be optimized in the test,because of the lower relative molecular mass of ammonia and formaldehyde.Meanwhile,the low solubility of hydrogen sulfide in kinds of solvents makes it be tested only by detector tube method.
1.1 Materials
The blank sample,cotton interfacing,bamboo-carbon fabric with silver ions,bamboo-carbon fabric,and hemp stocking were chosen to study the test method of the fabric deodorization,and were indicted by 1,2,3,4,and 5 respectively:1-blank sample,2-cotton interfacing(Shanghai Textile Industry of Technical Supervision,China),3-bamboocarbon fabric with silver ions(Wuxi Paiho Textile Ltd.,China),4-bamboo-carbon fabric(Wuxi Paiho Textile Ltd.,China),5-hemp stocking(Donghua University,China).
Ammonia/nitrogen standard substance:the concentration of ammonia for 75.9 mg/m3under 9.5 MPa,produced by ShanghaiWeichuang Standard Gas Co.,Ltd.,China;formaldehyde/nitrogen standard substance:the concentration of formaldehyde for 133.9 mg/m3under 9.5 MPa,produced by Shanghai Weichuang Standard Gas Co.,Ltd.,China;hydrogen sulfide/nitrogen standard substance:the concentration of hydrogen sulfide for 151.8 mg/m3under 9.5 MPa,produced by Shanghai WeichuangStandard Gas Co.,Ltd.,China;ethanol alcohol:analytically pure,produced by Sinopharm Chemical ReagentCo.,Ltd.,China;ammoniasolution:analytically pure,28%by weight,produced by Shanghai Anpu Scientific Instrument Co.,Ltd.,China;formaldehyde solution:chromatographically pure,concentration for 1.0 g/L,produced by Shanghai Anpu Scientific Instrument Co.,Ltd.,China.
1.2 Equipment
Tetrafluoroethylene air bag:5 L,produced by America Tedlar Company.
Hydrogen sulfide gas detector tube:measuring range 0.4-182.1 mg/m3,produced by Japan GASTEC Company.
Ammonia gas detector tube:measuring range 0.4-59.2 mg/m3,produced by Japan GASTEC Company.
Formaldehyde gas detector tube:measuring range 2.7-133.9 mg/m3,produced by Japan GASTEC Company.
GC-FID:Agilent 7890,produced by America Agilent Technology Company.
Gas flow meter:model of RK-1650,range of 0.3-3.0 L/min,produced by Japan KOFLOC Company.
1.3 Detector tube method
Put the prepared sample into the empty air bag and seal up the bag.Open the valve,fill in 3 L ammonia standard gas and close the valve.After adsorption,extract the amount of odorous substance(operating in Fig.1)then testthe residual concentration of ammonia once an hour and put down the reading (detectortube in Fig.2). Testthe residual concentration ofhydrogen sulfide and formaldehyde after adsorption with the same approach on ammonia.

1.4 GC-FID method
The sample has been prepared(as shown in Fig.3).And 4×10-6L ammonia solutions are put into the conical flask along the wall by syringe and the conical flask is sealed up.At 25 ℃,the solutions gasify and form into gas.After adsorpting ammonia by the sample,test the residual concentration of ammonia once an hour.Test the residual concentration of formaldehyde by the same way.

Fig.3 GC-FID samples
2 Results and Discussion
2.1 Analysis of detector tube method
2.1.1 The deodorizing properties of samples for ammonia
In daily life,the irritating smell of ammonia is the main source of dirty smell from organism decomposition,body,bedding,fecaluria,and smoking.So the ammonia was chosen as the source gas.
Figure 4 indicates the change of residual concentration of ammonia after odor's being adsorbed by cotton interfacing varies with time.The residual concentration of ammonia decreases gradually with the first 2 h.However,2 h later,the residual concentration of ammonia makes an insignificant change and the adsorption reaches a balance.So we choose 2 h as the adsorbing time when using the detector tube method in testing the deodorizing rate of fabric.
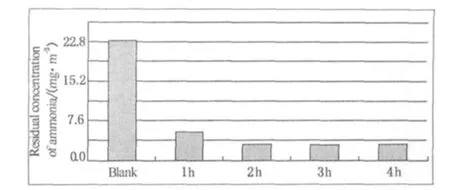
Fig.4 Residual concentration of ammonia after odor's being adsorbed by cotton interfacing
The ammonia residual concentrations of all samples after adsorbing are shown in the Fig.5.It can be seen that,the fabric samples have the deodorization for ammonia somewhat,and the deodorizing rates are all higher than 50%.
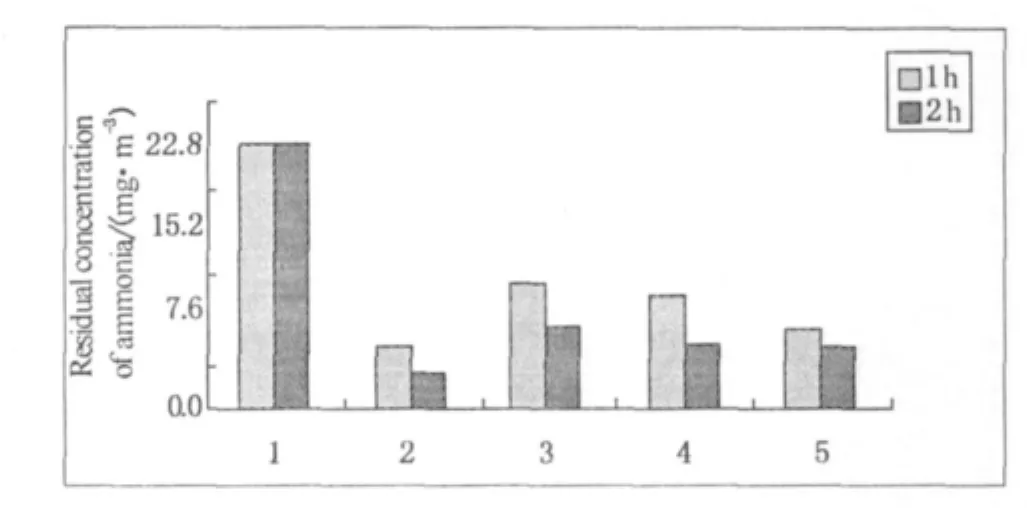
Fig.5 Residual concentration of ammonia after odor's being absorbed by samples
2.1.2 The deodorizing properties of samples for hydrogen sulfide
In our daily life,the odor of waste and the corruption smell of household garbage are inevitable.The main component of these odors is hydrogen sulfide,such as the odor of rotten eggs.Especially in hot days,the odor may bother people's normal life.If the fabric possesses the deodorization for hydrogen sulfide,that's good for the whole society.Therefore,it is important to find a way to calculate the deodorizing rate of fabric for hydrogen sulfide.
Figure 6 shows the residual concentration of hydrogen sulfide after adsorption of different samples.The samples above have a certain degree function of deodorization for hydrogen sulfide,which is weaker than that for ammonia.

Fig.6 Residual concentration of hydrogen sulfide after odor's being absorbed by samples
2.1.3 The deodorizing properties of samples for formaldehyde
Figure 7 shows that the fabric samples above have a certain degree ofdeodorization forformaldehyde.After2 h of adsorption,the deodorizing rates are mostly higher than 50%.Hemp stocking and cotton interfacing have better deodorizations for formaldehyde than others.

Fig.7 Residual concentration of formaldehyde after odor's being absorbed by samples
It is found that during testing the deodorizing rate by detector tube,the adsorption ability for ammonia is stronger than that for formaldehyde and hydrogen sulfide,which means the adsorption of samples on different odors is diverse.
Meanwhile,it's easy to operate and the reading of concentration is intuitive with lower accuracy when we use the detector tube method to test the residual concentration of odorous substance.
2.2 Analysis of GC-FID method
2.2.1 Deodorizing properties of samples for ammonia
To investigate the regularity change of ammonia without elapsing under the test condition in this paper,sealed ammonia is drawn out once an hour and its concentration is tested by GCFID.From Fig.8,we can see that under above-said test condition,there's no significant change of ammonia concentration which means the experimental system can ensure the sealing of odor-gas and the results aren't influenced by the volatilization and leakage of the gas.
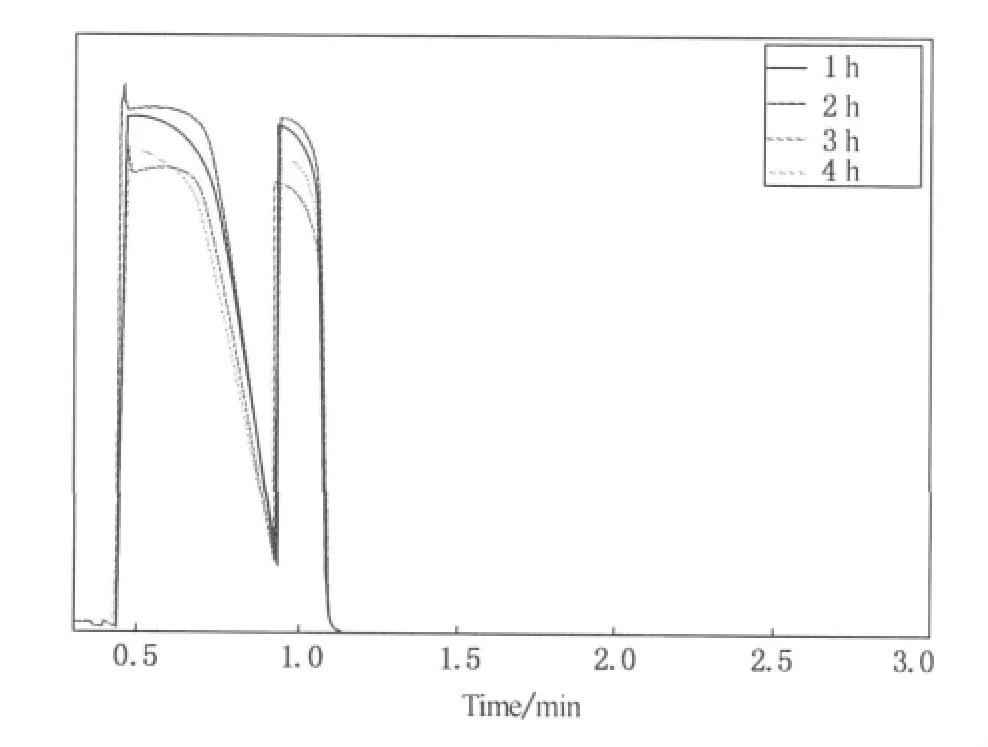
Fig.8 Blank of ammonia
The adsorptions ofcotton interfacing,bamboo-carbon fabric with silver ions,bamboo-carbon fabric,and hemp stocking for ammonia are shown in Table 1.The results show that all samples possess adsorption for ammonia.Furthermore the performance to clear ammonia up of cotton interfacing and bamboo-carbon fabric are better than that of bamboo-carbon fabric with silver ions,and hemp stocking.From Table 1,the residual concentration of ammonia after 1 h of adsorbing are basically stable,therefore,the adsorbing time is determined to be 1 h during the test of the deodorizing ammonia.
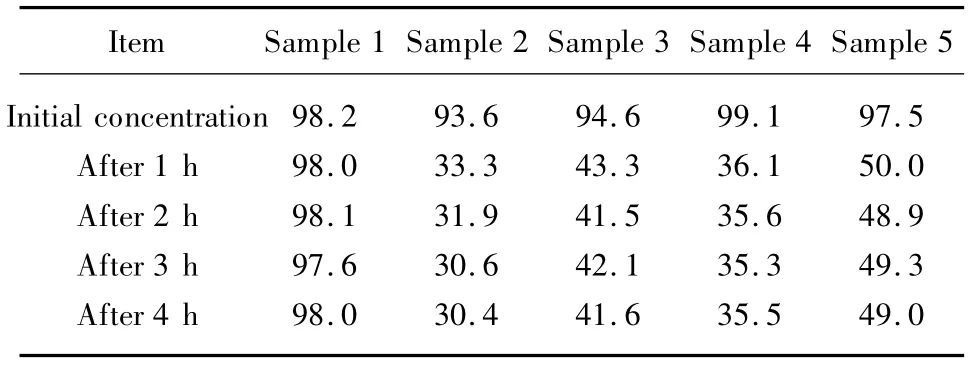
Table 1 The deodorizing properties of various samples for ammonia
2.2.2 Deodorizing properties of samples for formaldehyde
The adsorptions of various samples are shown in Table 2.The results show that the residual concentration of formaldehyde with different samples declines heavily after adsorbing for 1 h,and the residual concentration of the formaldehyde does not basically change as time prolongs.It can be seen from Table 2,the performance to eliminate formaldehyde of the hemp stock is a little better than that of other samples,thus the hemp stock can bring more comfort in the daily life.
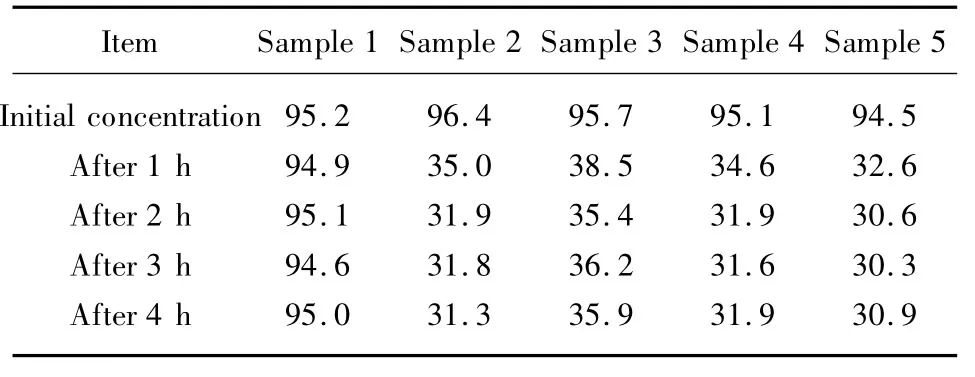
Table 2 The deodorizing properties of various samples for formaldehyde
3 Conclusions
(1)It is easy and quick to test the deodorization of fabric by using the detector tube method.The method can test the concentration of different single odorous substance,providing timely and accurate field monitoring data.Furthermore,it is a test approach with which the concentration readings can be read directly.The method is appropriate for testing the deodorization of fabric.
(2)Theoretically,the mixed substances can be tested by GC-FID method on deodorization of fabric.This kind of method also applies to testing the deodorization of fabric as detector tube method with sensitive analysis and high accuracy.
[1]Yu Z C,Chen W X,Chen H X.Research on Deodorizing Properties and Mechanism of Cellulose with Metal Phthalocyanine[J].Journal of Textile Research,2002,26(6):22-23.(in Chinese)
[2]Chen W X,Zhang L,Yao Y Y,et al.Preparation of Viscose FiberSupported-MetalTetracaboxyphthalocy Anine and Its Deodorizing Ability[J].Acta Polymerica Sinica,2006,12(9):1069-1073.(in Chinese)
[3]Gu H.The Developing of Photocatalysis Deodorization and Antibiosis Functionality Furnishing Fabric [J]. Knitting Industries,2005(5):25-28.(in Chinese)
[4]Zhao Z R,Ma Z S.Japanese Deodorization Technique and Its Application on Acrylic Fiber[J].Shandong Textile Economy,2006,133(3):69-73.(in Chinese)
[5]Wang D R.The Developing and Progress of Antibiosis and Deodorization[C].The Proceedings of the 8th Functional Textiles and Nano Technology Conference Symposium,Ningbo,2008:177-181
[6]Liu R H.The Developing on Deodorization of Fabric[J].Jiangsu Textile,2004(10):46-47.(in Chinese)
[7]Jie H.The Study on Deodorization and Antibiosis of Fabric with Modified Nano-TiO2Finishing[D].Hangzhou:Zhejiang Sci-Tech University,2008.(in Chinese)
[8]Mormann W,Wagner T. Silylation of Cellulose with Hexamethyldisilazane in Liquid Ammonia[J].Carbohydrate Polymers,2000,43(3):257-262.
[9]Koch P A.Lyocell Fibers-Alternative Regenerated Cellulose Fibers[J].Chemical Fibers International,1997,47:298-304.
[10]Chen W,Li Y H,Jin X K,et al.Wearing and Deodorant Property Study of the Fabric Blended with Photocatalyst Fiber and Cotton[J].Silk,2011,48(10):21-23.(in Chinese)
[11]Ji Rong K C.Odour Determination and Smell Sensor[J].Environmental Protection Science,2007,33(4):70-72.(in Chinese)
[12]Wang Z X.The Infrared Spectrometric Analysis and Identification of Polymer[M].Chengdu:Sichuan University Press,1989:45-48.(in Chinese)
[13]Park Y H,Nam Y J.The Antibacterial Activity and Deodorization of Fabrics Dyed with Lithospermi Radix Extract[J].Journal of the Korean Soiety of Clothing &Textiles,2003,127(1):60-66.(in Korean)
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Frost Resistance and Damage Velocity of Compressively Preloaded Concrete
- Vehicle OHT DispatchingPerformance Analysis ofan AMHS in 300mm Semiconductor FABs
- Research on Cooperation Mechanism ofChina'sFinancialRegulation and Supervision System
- Determination of the Dose of PAC in Ultrafiltration System for Drinking Water Treatment
- Hydrophobic TixOy-CmHnNanoparticle Film Prepared by Plasma Enhanced Chemical Vapor Deposition
- Sorption Kinetics and Capacity of Composite Materials Made up of Polymeric Fabric and Expanded Perlite for Oil in Water
