Na促进的CuCoMn催化剂催化生物质合成气合成高醇
2011-11-30叶同奇张朝霞颜世志朱九方李全新
叶同奇 张朝霞 徐 勇 颜世志 朱九方 刘 勇 李全新,*
(1中国科学技术大学化学物理系,生物质洁净能源安徽省重点实验室,合肥230026; 2合肥天焱绿色能源开发有限公司,合肥230026)
Na促进的CuCoMn催化剂催化生物质合成气合成高醇
叶同奇1张朝霞1徐 勇1颜世志1朱九方1刘 勇2李全新1,*
(1中国科学技术大学化学物理系,生物质洁净能源安徽省重点实验室,合肥230026;2合肥天焱绿色能源开发有限公司,合肥230026)
研究钠促进的CuCoMn催化剂的特性及其在生物质气化合成气合成高醇中的应用.研究了催化剂中Na含量及合成条件(温度、压力和空速)对生物质基合成气合成高醇性能的影响.发现CuCoMnNa0.1催化剂较适合高醇合成,在300°C以下,随着温度的上升,碳转化率增大,而醇选择性降低.压力的增加有利于醇的合成,增大空速会明显降低碳转化率,但醇时空产率则因转换频率的增加而增大.在所考察的范围内,醇产率最高达到304.6 g·kg-1·h-1,其中C2+高醇(C2-C6醇)占64.4%(w,质量分数).醇产物和烃产物均符合ASF(Anderson-Schulz-Flory)分布关系.根据催化剂性能与表征分析,Na的加入有利于提高生物质气化合成气合成高醇的选择性和活性元素Cu、Co的分散性.X射线光电子谱(XPS)测试结果显示反应后的催化剂表面上,Cu以Cu+和Cu0的混合形式存在,而Co则是Co2+/Co3+和Co0的混合物.增加Na的含量,Cu0/Cu+比率和Co0的强度均随之减小.
生物质;生物质合成气;高醇;ASF分布;CuCoMnNa催化剂
1 Introduction
With the gradual depletion of fossil fuel resources,increasing energy demand and global climate change,renewable energy such as biomass energy will play a more important role in the future energy scenario of the world.1Based on the thermochemical and biochemical processes,biomass can be converted into a wide range of liquid fuels(called as bio-fuels)or chemicals,such as bio-oil,bio-ethanol,bio-diesel,mixed alcohols,dimethyl ether(DME),etc.2,3However,the raw bio-oil from various biomass by pyrolysis processes can not be directly used in gasoline or diesel engines because of low heating value,poor volatility,high viscosity,coking,corrosiveness,and high water content,and it must be upgraded prior to being used as a replacement for diesel and gasoline fuels.4Another important route for the conversion of biomass to fuels is through its conversion to an intermediate synthesis gas,a mixture of CO and H2named bio-syngas.Bio-syngas can be further catalytically converted into various bio-fuels and chemicals,especially to methanol,ethanol,mixed alcohols,and Fischer-Tropsch(FT) fuels.5The unstinted feedstock type of biomass is one major advantage of this synthesis route.6,7
With stringent restrictions on pollution emissions,alcohols appear to not only be environmentally friendly fuel additives, but also effective as potential octane number enhancer for motor fuels.As a potential alternative fuel/additive or chemical raw materials,the higher alcohols have many advantages including complete combustion,higher octane numbers,volatility control,lower toxic exhaust gas(CO,NOx)emissions,excellent substitutes for methyl tert-butyl ether(MTBE)and higher added value.8,9So the catalytic conversion of synthesis gas to higher alcohols is now attracting renewed attention for both industrial application and fundamental research.
A wide range of homogeneous and heterogeneous catalysts for higher alcohol synthesis from syngas have been explored and well reviewed in recent papers.10These catalysts can be broadly classified into four types:noble metals-based catalysts,11-13modified Fischer-Tropsch catalysts,14-17modified methanol catalysts,18-20and Mo-based catalysts.21,22Among those alcohol synthesis catalysts,copper modified Fischer-Tropsch catalysts have drawn a wide attention because of their high activity and selectivity,such as CuCo-based catalysts developed by Institut Francais du Petrole.23For CuCo-based catalysts,cobalt was thought to provide the chain growth,while copper would be responsible for chain termination to produce alcohols.14The component synergism result from interaction between Cu and Co plays a very important role in alcohol synthesis.16However,the fact that these catalysts can be modified to increase their selectivity to higher alcohols suggests that they need to be further studied.
Generally speaking,a catalyst active for higher oxygenate synthesis must contain both adsorbed molecular CO,and surface carbon species derived from dissociative adsorption of CO.So the catalyst must be able to balance the CO dissociation and CO insertion that is necessary for the synthesis of higher alcohols.24Thus besides copper and cobalt,a third component mainly including transition metal elements(such as Zn, Cr,Mn,etc.)and a forth promoter of alkaline metal are also needed,14,15although their functions are remained not quite clear.In the past decades,many attentions have been paid to the Al,Cr,and/or Zn promoted CuCo catalysts,including the preparation methods,25alkali promote effects,15and catalytic performances.26Some new types of CuCo-based catalysts such as CuCo/CNTs have also been reported.27However,to the best of our knowledge,alkali-promoted CuCoMn catalysts for higher alcohol synthesis are rarely reported.
In our previous work,attention has been paid to produce syngas from the biomass gasification and the bio-oil reforming, both in lab and pilot plant scales.28-30Present work aims to efficiently produce higher alcohols over the CuCoMn-based catalyst from the bio-syngas.Moreover,the influences of sodium addition on catalyst structure and catalytic performance of alcohol synthesis were also investigated.Biomass gasification-synthesis route could produce higher alcohols through the use of any biomass resource in large quantities.However,the bio-syngas conversion to higher alcohols remains challenging,and no commercial process exists so far although there is a growing worldwide interest in this topic for the past decades.Further researches and developments in catalyst and processing need to be achieved to make this conversion commercially attractive.
2 Experimental
2.1 Catalyst preparation and characterization
The CuCoMn-based mixed oxides catalysts with a settled molar ratio(nCu:nCo:nMn=1:1:1)were prepared by the co-precipitation method from two aqueous solutions,one of which contained metal nitrate solution(A.R.)and the other contained sodium carbonate solution(A.R.).The metal nitrate solution was added quickly to the sodium carbonate solution at about 70°C. The coprecipitates were left to age in the mother liquor for 1 h, dried at 120°C for 12 h,and calcined at 450°C for 4 h in air to obtain the corresponding mixed oxide catalysts.The mixed oxides catalysts were finally crushed into 40-60 mesh for the higher alcohol syntheses.Catalysts before calcination were impregnated with different amounts of Na2CO3(A.R.)for CuCoMnNa0.1and CuCoMnNa0.2.The molar ratios of Cu/Co/ Mn/Na are 1:1:1:0.1 and 1:1:1:0.2 for CuCoMnNa0.1and CuCoMnNa0.2,respectively.
The Brunauer-Emmett-Teller(BET)surface area and pore volume were determined by the N2physisorption at-196°C using a COULTER SA 3100 analyzer.The X-ray diffraction (XRD)was measured on an X′pert Pro Philips diffractrometer with a Cu Kαradiation(λ=0.154 nm).The measurement conditions were in the range of 2θ=10°-80°,step counting time 5 s, and step size 0.017°at 25°C.The surface elements and their states were analyzed by X-ray photoelectron spectroscopy (XPS).The XPS measurements were performed on an ESCALAB-250(Thermo-VG Scientific,USA)spectrometer with Al Kα(1486.6 eV)irradiation source.The C 1s peak at 284.6 eV was generally used as a calibration standard for determining the peaks′position and the elemental concentration.
2.2 Reaction system for higher alcohol syntheses
As shown in Fig.1,the performance of higher alcohol syntheses from the selected bio-syngas over the different CuCoMnbased catalysts was evaluated in a fixed-bed continuous-flow reactor using an on-line gas chromatograph(GC)detection system.The cylindrical reactor was constructed from 316L with 40 cm length and an internal diameter of 1 cm.Gas flow rates were regulated using Seven Star 17B mass flow controllers.Reactor pressure was maintained by a back pressure regulator. The catalyst bed temperature was measured during reactions using a type K thermocouple positioned within the reactor itself,near the center of the catalyst bed.
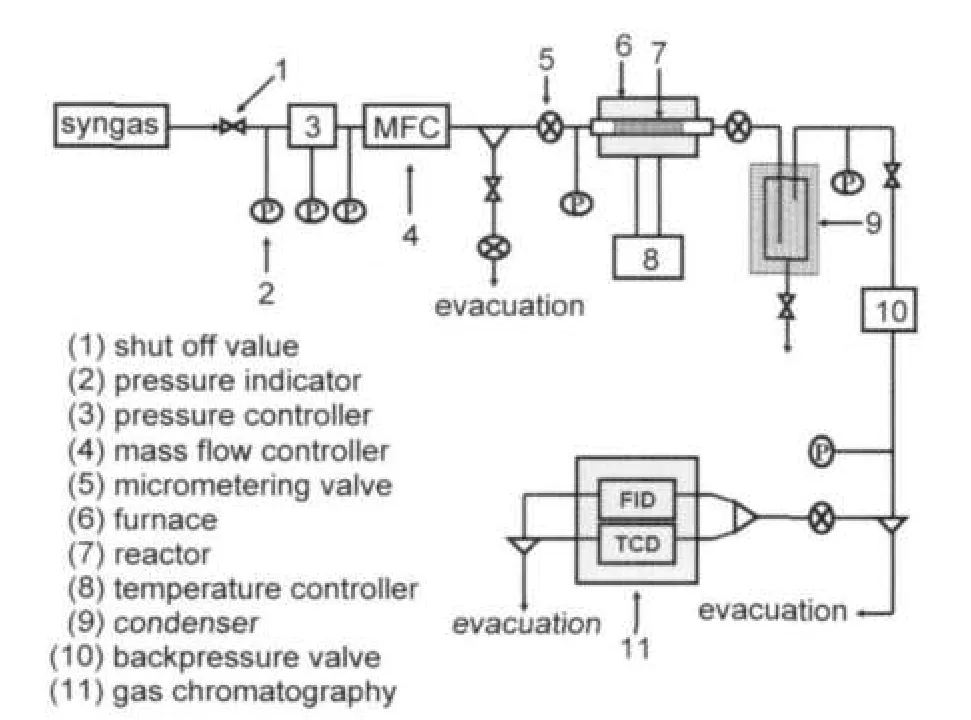
Fig.1 Schematic setup of the fixed-bed flow reaction system for mixed alcohol synthesis
Usually,1.0 mL catalyst,diluted with 2.0 mL Pyrex beads, was loaded in the reactor in any cases.Prior to kinetic tests the catalysts were activated with 5%(volume fraction)H2/Ar at 320°C for 12 h.Then,bio-syngas was conducted to the reactor for the higher alcohol syntheses under a setup synthesis condition.The syntheses were carried out under typical operating conditions:T=260-320°C,p=3.0-7.0 MPa,GHSV(gas hourly space velocity)=3000-9000 h-1.Quantitative product analysis from the reactor outlet stream was on-line sampled every 15 min using two on-line gas chromatographs(GC1 and GC2). The gases of H2,CO,and CO2were detected by GC1(Model: SP6890,column:TDX-01)with a thermal conductivity detector(TCD),and gaseous hydrocarbons were detected by GC2 (Model:SP6890,column:PorapakQ-S,USA)with a flame ionization detector(FID).The condensable vapors(mainly consisting of higher alcohols and water)were cooled into a liquid tank and then detected offline by GC2 with a FID.The performance of higher alcohol syntheses was evaluated by the carbon conversion(CC),space time yield of higher alcohols(YAlc,g· kg-1·h-1),selectivity of alcohols(SAlc),and hydrocarbons(SHc), according to the following equations:

2.3 Feedstock for higher alcohol syntheses
In this work,one bio-syngas derived from the biomass gasification was used for the higher alcohol synthesis.The bio-syngas was produced by biomass gasification in a circulating fluidized bed using rice husks with the gasification temperature of 1000-1300°C and pressure of 1.5-3.0 MPa,followed by conditioning the syngas via water-gas shift(WGS)reaction and purification processes.31The main composition of the bio-syngas is H262.80%,CO 30.89%,CO22.96%,N21.75%,CH41.20%, and others 0.40%(volume fraction).
3 Results and discussion
3.1 Catalyst screening
Catalysts were prepared with the sodium promoter concentration varied from 0 to 6%(molar fraction)and compared under constant conditions of 5.0 MPa,300°C and gas hourly space velocity(GHSV)of 6000 h-1.The CO hydrogenation performances summarized in Table 1 show that the catalyst with a 3%(molar fraction)Na loading produces the highest alcohol synthesis activity.The overall activity of the promoted catalyst increases as the Na loading increases from 0 to 3%,showing the highest carbon conversion of 36.2%,while decreases rapidly to 24.2%when the Na loading further increases to 6%.As shown in Table 1,Na promoter not only influences catalytic activity,but also promotes alcohol selectivity and inhibits hydro-carbon synthesis in CO hydrogenation reaction.The total alcohol selectivity(SC)increased from 24.3%to 46.8%as Na loading(xNa)increased from 0 to 3%,while the hydrocarbon selectivity decreased from 51.7%to 20.5%,respectively.

Table 1 Performance of higher alcohol synthesis for various sodium loading
Alkali addition has been demonstrated to influence the catalyst activity through two typical ways.One is to facilitate adsorption of CO molecules on the catalyst surface16,32thus resulting in a higher efficiency of hydrogenation,as the 3%Na loading catalyst shows.The other is to cover the active sites which induce a decrease of catalytic activity.As Boz14reported that the addition of K to the CuCoZnAl catalyst resulted in the suppression of overall catalytic activity on higher alcohol synthesis.And Chen et al.33proved the excessive K2O loading induced a serious accumulation of potassium on the catalyst surface by XPS characterization.The presence of Na additive strongly suppresses the formation of hydrocarbons(Table 1),possibly due to the decrease in the availability of H*atoms required for termination of growth chains via hydrogen addition reactions to produce paraffins.34,35However,another view proposed by Courty et al.36for the enhanced alcohol selectivity is that alkali addition suppresses alcohol dehydration by suppressing the acidic nature of the catalyst.
3.2 Performance of higher alcohol synthesis
Table 2 shows the influence of operating conditions(temperature,pressure,and GHSV)on the higher alcohol synthesis using bio-syngas over the 3%Na promoted catalyst under the synthesis conditions:T=260-320°C,p=3.0-7.0 MPa,and GHSV=3000-9000 h-1.Commonly,temperature is one of the most critical reaction parameters in the higher alcohol synthesis,which significantly affects the rate of kinetically controlled synthesis reactions.In the lower temperature region,an increasing temperature is conducive to the dissociative adsorption of CO and H2while promoting the formation of the specified intermediates(e.g.,alkyls and formyl species),37,38which leads to an increase of the CO hydrogenation.However,another important characteristic of higher alcohol synthesis is the unavoidable production of a large amount of hydrocarbons,and exorbitant temperature will greatly decrease the alcohol selectivity and enhance the formation of hydrocarbons.39,40Consequently, considering the balance of productivity and selectivity,an appropriate temperature needs to be determined through experimentation and then to be closely controlled at this value in the reactor.
As shown in Table 2,the carbon conversion significantly increased from 11.9%to 59.7%with a rising temperature from 260 to 320°C.An increasing trend was also observed for the space time yield of higher alcohols in this range.The selectivity towards total alcohols(C1-C6alcohols)decreased from 61.8%to 28.3%with a rising temperature versus an opposite trend for the hydrocarbons selectivity.In the hydrocarbon distribution,products were almost C1-C4gaseous hydrocarbons besides a small quantity of liquid hydrocarbons.In the alcohols products,the C2+alcohols(C2-C6higher alcohols)contained with a mass fraction of 28.7%-63.9%(w)and main alcohol products were methanol,ethanol,and propanol under the tested synthesis conditions.When the temperature was fixed at 300°C,the carbon conversion and space time yield ascended monotonously as pressure increased,for the synthesis reactions involved a decrease in the number of molecules.In contrast, the carbon conversion decreased with the increase of the gas hourly space velocity,which was accompanied by an increase of the space time yield of higher alcohol.The negative impact of GHSV on the carbon conversion may result from shortening residence time in the catalyst bed,while the positive impact on the fuel yield can arise from the increase of the turnover frequency of the synthesis products with increasing GHSV.The maximum higher alcohol yield from bio-syngas was about 304.6 g·kg-1·h-1with the alcohol selectivity of 50.1%and C2+alcohols distribution of 64.4%(w)within our studied range. Apart from the alcohol and hydrocarbon products,only a small amount of other compounds including aldehydes,ketones,esters,and ethers were also detected.
Moreover,the catalytic stability in the higher alcohol synthesis process was tested by measuring the CO conversion,selectivity of alcohols and hydrocarbons,yields of alcohols as a function of time on stream.As shown in Fig.2,the activity increases initially until it reaches the maximum at about 4-6 h of time on stream,and then it decreases very slowly in our tested 80 h.The selectivity of hydrocarbons increases gradually,however,both the alcohol selectivity and yields follow a trend of decrease.A long-term(80 h)reaction test led to about 5%-8% reduction in the higher alcohol synthesis activity compared tothe maximum values.Generally,the slow catalyst inactivation observed in the higher alcohol synthesis process could mainly ascribe to the sintering of active sites and deposition of the carbon16on the catalysts.
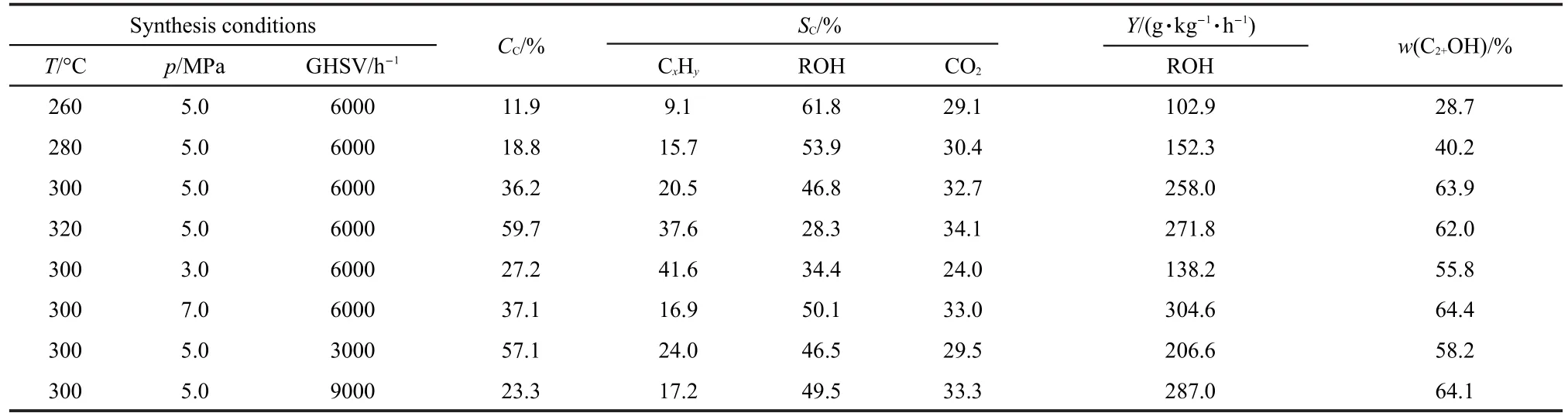
Table 2 Performance of higher alcohol synthesis using bio-syngas over CuCoMnNa0.1catalyst

Fig.2 Stability of the CuCoMnNa0.1catalyst in the higher alcohol synthesis(a)yield of higher alcohols,(b)selectivity of alcohols,(c)carbon conversion, (d)selectivity of hydrocarbons;synthesis conditions:T=300°C, GHSV=6000 h-1,p=5.0 MPa
As shown in Fig.3,both alcohols and hydrocarbon products were consistent with the ASF distributions.41Interestingly,the rate of methanol formation was also in line with higher alcohols according to the ASF distribution.Using the ASF distribution we determined the chain growth probability(α)from the slope of the linear part of the plot.The α values of alcohols were found to be increased(from 0.196 to 0.362)while those of hydrocarbons remained nearly constant(α=0.460±0.020)for the CuCoMnNa0.1catalyst when temperature increased from 260 to 300°C.This fact can be taken as an indirect evidence that alcohols and hydrocarbons were formed on different active sites for CuCo-based catalysts as Boz14proposed.
3.3 Catalyst characterization

Fig.3 Anderson-Schulz-Flory plots of(a)hydrocarbons and(b)alcohols over CuCoMnNa0.1catalyst at different temperatures Wnis the mass fraction of a product containing n carbon atoms.
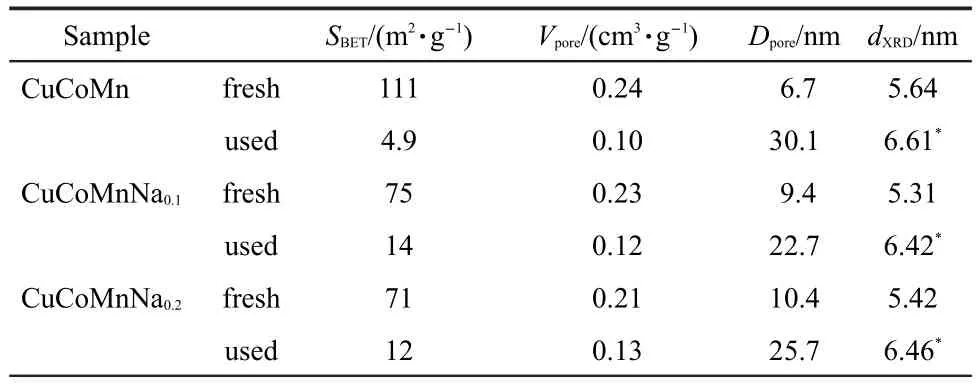
Table 3 Texture parameters of fresh and used CuCoMn-based catalysts
Some of important physical and chemical properties,including BET surface area,pore volume,and the size of the crystallites were investigated for the CuCoMn-based catalysts before and after used.As shown in Table 3,the doping of sodium in the catalyst induced an obviously decrease of BET surface area and increase of pore size.The BET surface area decreased from 111 to 71 m2·g-1,while the pore size increased from 6.7 to 10.4 nm with the Na loading from 0 to 6%.Such an effect may be related to the clogging of micropores caused by sodium carbonate when considering the adsorption/desorption isotherm data.16
Fig.4 shows the XRD patterns of the fresh CuCoMnNa0.1catalyst,the reduced ones(pure H2,320°C,8 h),and the used ones(T=300°C,p=5.0 MPa,GHSV=6000 h-1,t=20 h)for higher alcohol synthesis,corresponding patterns of CuCuMn catalyst were also shown.For the fresh samples,all the diffraction peaks can be assigned to spinal CuCoMnO4(JCPDS 47-0324).42Formation of the mixture oxide may be the cause of the excellent catalytic stability of CuCoMn catalysts.The addition of Na promoter did not present significant structural modification when compared with the diffractogram of none sodium addition sample.As suggested by Dalmon et al.16for the CoAl-based catalysts,alkali doping probably just coats the surface as sodium carbonate.For the catalysts reduced by H2, Cu(JCPDS 04-0836)and MnO(JCPDS 01-1206)were detected,but none of Co signals was found suggesting a high dispersion of Co species under reduction conditions.However,after being used at 300°C for higher alcohol synthesis,the diffraction peaks of Co3O4appeared probably due to a slightly sintering.Moreover,the Cu particle sizes for the used catalysts calculated by XRD line widths of the strongest peak(2θ=43.4°) using Debye-Scherrer equation are shown in Table 3.Compared with the none-sodium addition catalyst,the Na-promoted sample showed a smaller Cu particle size.It is well-known that catalysts with small crystallite sizes have an advantage to produce more alcohols while larger crystallites to hydrocarbons.43
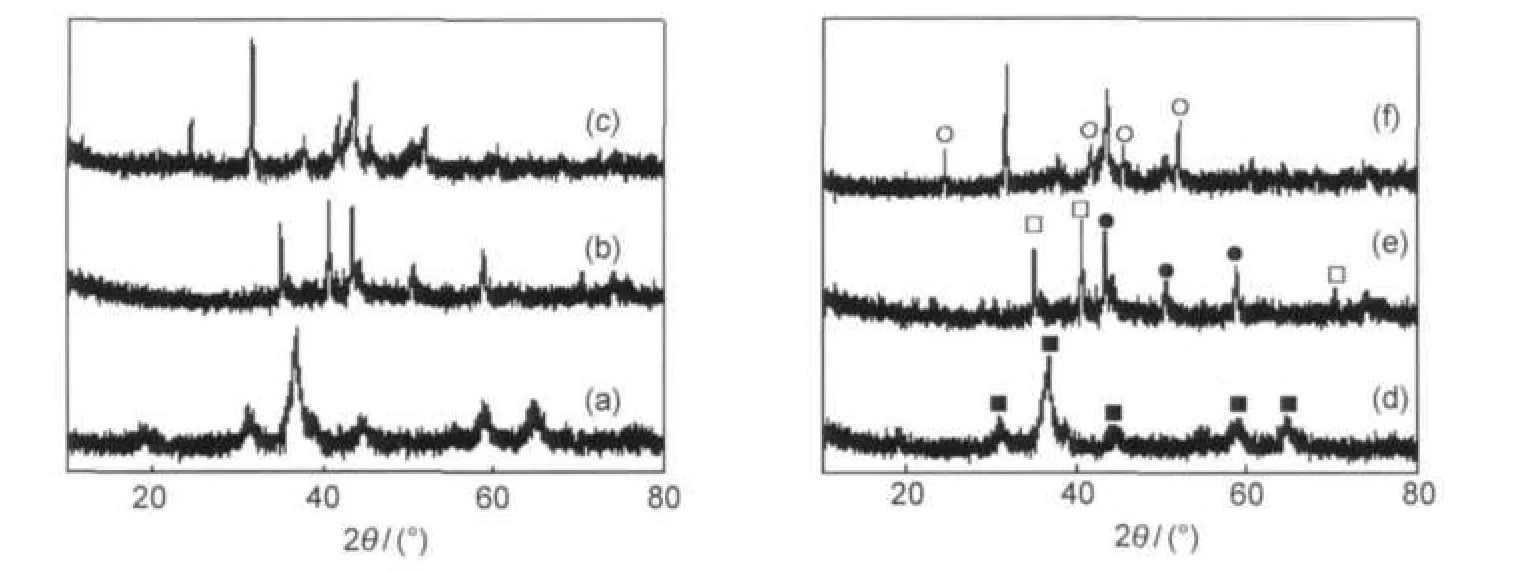
Fig.4 XRD patterns of(a)fresh CuCoMn catalyst,(b)reduced CuCoMn catalyst,(c)used CuCoMn catalyst, (d)fresh CuCoMnNa0.1catalyst,(e)reduced CuCoMnNa0.1catalyst,and(f)used CuCoMnNa0.1catalyst■CuCoMnO4,●Cu,□MnO,○Co3O4;synthesis conditions:(b)T=300°C,pure H2,p=0.5 MPa,(c)T=300°C,GHSV=6000 h-1,p=5.0 MPa,t=20 h, (e)T=300°C,pure H2,p=0.5 MPa,(f)T=300°C,GHSV=6000 h-1,p=5.0 MPa,t=20 h
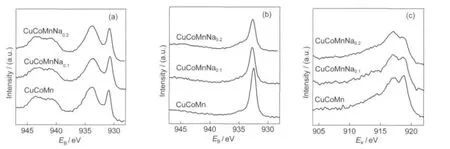
Fig.5 Cu 2pXPS spectra and Cu LMMAuger electron spectra for different catalysts (a)Cu 2p XPS spectra for fresh catalysts,(b)Cu 2p XPS spectra for used catalysts,(c)Cu LMMAuger electron spectra for used catalysts; synthesis conditions:T=300°C,p=0.5 MPa,GHSV=6000 h-1,t=20 h

Fig.6 Co 2p XPS spectra for different catalysts (a)Co 2p XPS spectra for fresh catalysts,(b)Co 2p XPS spectra for used catalysts;synthesis conditions:T=300°C,p=0.5 MPa,GHSV=6000 h-1,t=20 h
The alterations of the atomic states on the catalyst′s surfaces before and after the synthesis reaction were investigated by the XPS measurements.As can be seen from Fig.5(a),the binding energy at about 930.8 and 933.7 eV were observed for the pristine catalysts,which were assigned to the main line of Cu+(2p3/2) and Cu2+(2p3/2),44respectively.Note that no reduction was employed on the catalysts,the presence of Cu+should attribute to the internal reduction of Mn3+,which has been proved by Yang et al.42After the higher alcohol synthesis,the Cu2+on the surface was reduced as Fig.5(b)shows.For the Cu0and Cu+species can not be distinguished by the 2p3/2peak,Fig.5(c)shows the Cu LMM Auger electron spectra.It can be found that the used samples exhibit a double peak structure at kinetic energy values of 917.0 and 918.7 eV.According to Velu et al.,44these two peaks are corresponding to Cu+and Cu metal,respectively. With the increasing of sodium addition,the Cu+/Cu0ratio increased,indicating the stabilization of Cu+species.A similar internal reduction effect was also observed on Co as Fig.6(a) shows,the weak satellite of Co3+(2p2/3)was about 10 eV higher than its main peak,not as commonly 6 eV.In addition,the very weak satellite shows a mixture of Co2+and Co3+.44After the higher alcohol synthesis,as Fig.6(b)shows,a peak at binding energy(EB)value of 778.8 eV appeared,which could be assigned to Co0species.However,the intensity of Co0decreased with increasing the Na content.Some argue that Co0is an active site of hydrogenation that tends to formation of hydrocarbons.14
4 Conclusions
This work reports that higher alcohols can be efficiently produced from bio-syngas derived from the biomass gasification using Na-promoted CuCoMn catalysts.Appropriate amount of sodium enhances the total alcohol selectivity and productivity. CuCoMnNa0.1catalyst is moderately selective for production of higher alcohols under mild conditions.It was found that alcohol selectivity decreased monotonously with the temperature increasing,while the mass fraction of C2+(C2-C6)alcohols in total alcohol products increased.The optimum temperature was about 300°C based on the higher alcohol selectivity and productivity.The maximum higher alcohol yield from the biosyngas was about 304.6 g·kg-1·h-1with the alcohol selectivity of 50.1%and C2+alcohols distribution of 64.4%(w)within the tested conditions.Considering of the ASF distributions,there probably exists two different active sites with two distinctly chain growth probability factors for alcohols and hydrocarbons,respectively.According to XRD analysis,CuCoMnO4mixture oxide is the dominant phase for the fresh catalysts. XPS results suggest that Cu presents as mixture of Cu+and Cu0on the catalyst surface after being used,and Co presents as mixture of Co2+/Co3+and Co0.With increasing of sodium addition,the Cu0/Cu+ratio and the Co0intensity both decreased. The CuCoMnNa0.1catalyst may be one of the most suitable candidates for the higher alcohol synthesis from bio-syngas because this non-noble metal catalyst can efficiently produce higher alcohols through the hydrogenation of CO.The higher alcohols derived from bio-syngas with higher octane values could be used as transportation fuels or petrol additives.The bio-fuels synthesis is unstinted by the feedstocks of biomass, and potentially,may be one promising route to produce bio-fuels in future.
(1) Navarro,R.M.;Pena,M.A.;Fierro,J.L.G.Chem.Rev.2007, 107,3952.
(2) Zhang,Q.;Chang,J.;Wang,T.J.;Xu,Y.Energy Convers. Manage.2007,48,87
(3)Li,H.Y.;Xu,Q.L.;Xue,H.S.;Yan,Y.J.Renewable Energy 2009,34,2872.
(4) Czernik,S.;Bridgwater,A.V.Energy&Fuels 2004,18,590.
(5)Zhou,M.;Yan,L.F.;Wang,Y.Q.;Guo,Q.X.;Zhu,Q.S.Chin. J.Chem.Phys.2005,18,69.[周 密,闫立峰,王益群,郭庆祥,朱清时.化学物理学报,2005,18,69.]
(6)Tijmensen,M.J.A.;Faaij,A.P.C.;Hamelinck,C.N.;Van Hardeveld,M.R.M.Biomass Bioenergy 2002,23,129.
(7) Steen,E.V.;Claeys,M.Chem.Eng.Technol.2008,31,655.
(8) Xu,X.D.;Doesburg,E.B.M.;Sckolen,J.J.F.Catal.Today 1987,2,125.
(9) Verkerk,A.N.;Jaeger,B.;Finkeldei,C.H.;Keim,W.Appl. Catal.A 1999,186,407.
(10) Subramani,V.;Gangwal,S.K.Energy&Fuels 2008,22,814.
(11) Li,Z.R.;Fu,Y.L.;Jiang,M.;Hu,T.D.;Liu,T.;Xie,Y.N. Chin.J.Chem.Phys.,2001,14,355.[李忠瑞,伏义路,姜明,胡天斗,刘 涛,谢亚宁.化学物理学报,2001,14,355.]
(12)Zhang,W.;Luo,H.Y.;Zhou,H.W.;Wu,Z.H.;Huang,S.Y.; Liu,C.Z.;Chu,H.P.;Lin,P.Z.;Lin,L.W.Chin.J.Catal. 1999,20,285.[张 伟,罗洪源,周焕文,吴治华,黄世煜,刘崇早,初惠萍,林培滋,林励吾.催化学报,1999,20,285.]
(13) Ojeda,M.;Granados,M.L.;Rojas,S.;Terreros,P.; Garcia-Garcia,F.J.;Fierro,J.L.G.Appl.Catal.A 2004,261, 47.
(14) Boz,I.Catal.Lett.2003,87,187.
(15)Tien-Thao,N.;Zahedi-Niaki,M.H.;Alamdari,H.;Kaliaguine, S.J.Catal.2007,245,348.
(16)Dalmona,J.A.;Chaumetteb,P.;Mirodatos,C.Catal.Today 1992,15,101.
(17) Su,Y.L.;Liu,B.;Pei,S.P.;Wang,X.Y.;Liu,Z.M.Chin.J. Catal.2004,25,683.[苏运来,刘 博,裴素朋,王向宇,刘中民.催化学报,2004,25,683.]
(18)Xu,R.;Wei,W.;Li,W.H.;Hu,T.D.;Sun,Y.H.J.Mol.Catal. A 2005,234,75.
(19) Gupta,M.;Spivey,J.J.Catal.Today 2009,147,126.
(20)Chen,X.P.;Wu,G.S.;Wang,X.Z.;Sun,Y.H.;Zhong,B. Chin.J.Catal.2000,21,301.[陈小平,吴贵升,王秀芝,孙予罕,钟 炳.催化学报,2000,21,301.]
(21) Li,D.B.;Qi,H.J.;Li,W.H.;Sun,Y.H.;Zhong,B.Acta Phys.-Chim.Sin.2006,22,1132.[李德宝,齐会杰,李文怀,孙予罕,钟 炳.物理化学学报,2006,22,1132.]
(22) Ma,X.M.;Lin,G.D.;Zhang,H.B.Chin.J.Catal.2006,27, 1019.[马晓明,林国栋,张鸿斌.催化学报,2006,27,1019.]
(23) Sugier,A.;Freund,E.;Malmaison,R.Process for ManufacturingAlcohols and More Particularly Saturated Linear PrimaryAlcohols from Synthesis Gas.US Pat.Appl.105312, 1981.
(24) Spivey,J.J.;Kumar,C.S.S.R.;Balaji,G.;Subramanian,N.D. Catal.Today 2009,147,100.
(25) Xu,H.Y.;Chu,W.;Deng,S.Y.Acta Phys.-Chim.Sin.2010,26, 345.[徐慧远,储 伟,邓思玉.物理化学学报,2010,26, 345.]
(26) Mehr,J.Y.;Islami,M.;Peyrovi,M.H.;Mahdavi,V.Appl. Catal.A 2005,281,259.
(27) Zhang,H.B.;Dong,X.;Lin,G.D.;Liang,X.L.;Li,H.Y. Chem.Commun.2005,5094.
(28) Kan,T.;Xiong,J.X.;Li,X.L.;Ye,T.Q.;Yuan,L.X.; Torimoto,Y.;Yamamoto,M.;Li,Q.X.Int.J.Hydrog.Energy 2010,35,518.
(29)Yuan,L.X.;Chen,Y.Q.;Song,C.F.;Ye,T.Q.;Guo,Q.X.; Zhu,Q.S.;Torimoto,Y.;Li,Q.X.Chem.Commun.2008,5215.
(30)Ye,T.Q.;Yuan,L.X.;Chen,Y.Q.;Kan,T.;Tu,J.;Zhu,X.F.; Torimoto,Y.;Yamamoto,M.;Li,Q.X.Catal.Lett.2009,127, 323.
(31) Liu,Y.;Chen,F.;Zhuang,S.X.;Wang,J.J.;Ma,R.G. AMethod and Equipment for Preparation of Syngas from Solid Biomass.CN Patent CN101191060A,2007.[刘 勇,陈 枫,庄叔贤,王家俊,马仁贵.一种由固体生物质制备合成气的方法和设备:中国,CN101191060A[P],2007]
(32)Aquino,A.D.;Cobo,A.J.G.Catal.Today 2001,65,209.
(33) Chen,B.S.;Zhao,J.S.;Zhang,L.;Xiong,G.X.;Sheng,S.S. Chin.J.Catal.1990,11,265.[陈宝树,赵九生,张 鎏,熊国兴,盛世善.催化学报,1990,11,265.]
(34) Li,S.;Li,A.;Krishnamoorthy,S.;Iglesia,E.Catal.Lett.2001, 77,197.
(35) Mross,W.D.Catal.Rev.Sci.Eng.1983,25,591.
(36) Courty,P.;Durand,D.;Freund,E.;Sugier,A.J.Mol.Catal. 1982,17,241.
(37) Laan,G.P.V.;Beenackers,A.A.C.M.Catal.Rev.Sci.Eng. 1999,41,255.
(38)Sachtler,W.M.H.;Ichikawa,M.J.Phys.Chem.1986,90,4752.
(39) Dry,M.E.Catal.Today 2002,71,227.
(40) Huang,X.;Curtis,C.W.;Roberts,C.B.Fuel Chemistry Division Preprints 2002,47,150.
(41) Schulz,H.Appl.Catal.A 1999,186,3.
(42)Yang,B.L.;Chan,S.F.;Chang,W.S.;Chen,Y.Z.J.Catal. 1991,130,52.
(43) Li,D.B.;Yang,C.;Li,W.H.;Sun,Y.H.;Zhong,B.Top.Catal. 2005,32,233.
(44) Velu,S.;Suzuki,K.;Gopinath,C.S.J.Phys.Chem.B 2002, 106,12737
January 20,2011;Revised:April 1,2011;Published on Web:April 22,2011.
Higher Alcohol Synthesis from Bio-Syngas over Na-Promoted CuCoMn Catalyst
YE Tong-Qi1ZHANG Zhao-Xia1XU Yong1YAN Shi-Zhi1ZHU Jiu-Fang1LIU Yong2LI Quan-Xin1,*
(1Anhui Key Laboratory of Biomass Clean Energy,Department of Chemical Physics,University of Science and Technology of China, Hefei 230026,P.R.China;2Hefei Tianyan Green Energy Development Co.,Ltd.,Hefei 230026,P.R.China)
Na-promoted CuCoMn catalysts were successfully applied to the highly efficient production of higher alcohols from bio-syngas,which was derived from biomass gasification.The influence of Na content and synthesis conditions(temperature,pressure,and gas hourly space velocity(GHSV))on higher alcohol synthesis was investigated.The CuCoMnNa0.1catalyst gave the best performance for higher alcohol synthesis.Carbon conversion increased significantly with an increase in temperature at lower than 300°C but alcohol selectivity showed an opposite trend.A higher pressure was found to be beneficial for higher alcohol synthesis.Increasing the GHSV reduced carbon conversion but increased the yield of higher alcohols.The maximum higher alcohol yield that was derived from bio-syngas was 304.6 g·kg-1·h-1with the C2+alcohols(C2-C6higher alcohols)of 64.4%(w,mass fraction)under the conditions used.The distributions of the alcohols and the hydrocarbons were consistent with Anderson-Schulz-Flory(ASF)plots.Adding Na to the CuCoMn catalysts led to an increase in the selectivity toward the higher alcohols and promoted the dispersion of the active elements,copper and cobalt.X-ray photoelectron spectroscopy(XPS)results suggested that Cu was present as a mixture of Cu+and Cu0on the catalyst′s surface after use and Co was present as a mixture of Co2+/Co3+and Co0.With an increase in sodium addition the Cu0/Cu+ratio and the Co0intensity both decreased.
Biomass;Bio-syngas;Higher alcohol;ASF distribution;CuCoMnNa catalyst
O643
∗Corresponding author.Email:liqx@ustc.edu.cn;Tel:+86-551-3601118.
The project was supported by the National Natural Science Foundation of China(50772107),National Key Basic Research Program of China(973) (2007CB210206)and National High-Tech Research and Development Program of China(863)(2009AA05Z435).
国家自然科学基金(50772107),国家重点基础研究发展规划(973)(2007CB210206)及国家高技术研究发展计划(863)(2009AA05Z435)资助项目
