合成金鸡纳生物碱衍生物的简便方法*1
2011-11-23南鹏娟孙晓莉
南鹏娟, 陈 晶, 孙晓莉
(1. 陕西师范大学 化学与材料学院 陕西 西安 710062; 2. 第四军医大学 化学系,陕西 西安 710062)
锇催化的不对称双羟化(AD)反应及不对称氨羟化(AA)反应是2001年诺贝尔化学奖获得者Sharpless教授于1988年和1996年首次报道的两类重要的催化不对称反应[1,2]。AD和AA反应分别是合成手性连二醇和手性β-氨基醇的最重要的方法之一[3]。目前该反应已成功地应用于紫杉醇C13侧链、大环抗癌药物、氨苄青霉素、昆虫激素、维生素D活性代谢物等的合成[4~6]。影响这两种反应工业化的一个关键因素是手性配体,这也是近年来各国科学家研究的一个热点。经多年研究和筛选,在AD和AA反应中最优秀的手性配体仍然是Sharpless先后报道的金鸡纳生物碱衍生物[7~13]。这些被称为第二代的手性配体具有催化活性高,立体选择性好等优点,如何高效的得到这一类配体又成为另一个研究热点。
在对AD和AA反应研究的过程中,我们发现了一种新的缚酸剂CaH2,在较温和的条件下(90 ℃),以DMF作溶剂,金鸡纳生物碱ArOH(1a~1d, Chart 1)通过3,6-二氯哒嗪(2)或1,4-二氯-2,3-二氮杂萘(3)桥连,高产率地合成了13个金鸡纳生物碱衍生物(4a~5g, Scheme 1),其结构经NMR确证。
1 实验部分
1.1 仪器与试剂
XRC-1型显微熔点仪(温度计未经校正);PE 343型自动旋光仪;Varian INOVA-400型核磁共振波谱仪(CDCl3为溶剂,TMS为内标)。
奎宁(1c, No.011220),德国进口分装;NaH和CaH2, Merck-Schuchardt;其余试剂均为分析纯或化学纯。
1.2 合成
(1)4的合成通法
在三颈瓶中加入3,6-二氯哒嗪(2) 2.98 g(20 mmol),1a~1d40 mmol, CaH28.4 g(200 mmol), DMF 60 mL, N2保护,搅拌下于90 ℃~100 ℃反应12 h(TLC跟踪)。加入乙酸乙酯150 mL和水50 mL,搅拌均匀后静置分层,水层用乙酸乙酯(2×150 mL)萃取,合并有机层,用水(3×80 mL)洗涤,无水MgSO4干燥,减压浓缩后用乙酸乙酯重结晶得白色固体4a~4d。
1c或1d20 mmol, CaH24.2 g(100 mmol),其余反应条件同上制得白色固体4e或4f。
(2)5a~5d的合成
在三口瓶中加入1,4-二氯-2,3-二氮杂萘(3) 2.95 g(15 mmol),1a~1d30 mmol, CaH26.3 g(150 mmol), DMF 45 mL, N2保护,搅拌下于90 ℃~100 ℃反应1 h(TLC跟踪)。加入乙酸乙酯100 mL和水50 mL,搅拌均匀后静置分层,水层用乙酸乙酯(3×150 mL)萃取,合并有机层,用无水MgSO4干燥,减压浓缩至约3 mL,静置析晶,抽滤,滤饼用少许无水乙醚洗涤、干燥得白色晶体5a~5d。
1b,1c或1d15 mmol, CaH23.15 g(75 mmol),其余反应条件同上制得白色晶体5g,5e或5f。
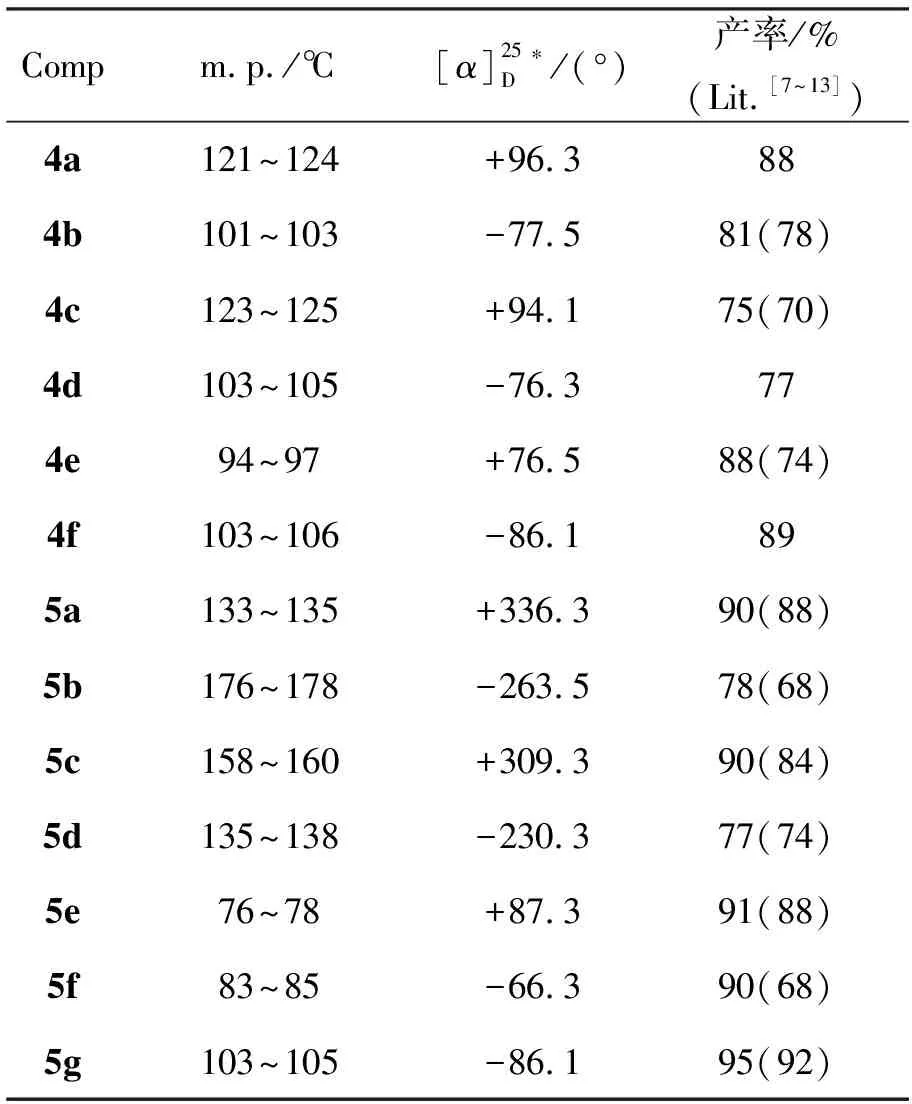
表 1 4a~5g的实验结果Table 1 The experimental results of 4a~5g
*4b:c1.0, MeOH,其余c1.0, CH2Cl2
4a~5g的实验结果见表1,表征数据[14]与Scheme 1预期吻合。
5c:1H NMRδ: 8.65(d,J=5.3 Hz, 2H), 8.31~8.33(m, 2H), 7.95~7.98(m, 4H), 7.58(s, 2H), 7.44(d,J=4.2 Hz, 2H), 7.38(m, 2H), 7.03(s, 2H), 5.78~5.86(m, 2H), 4.99(m, 4H), 3.93(s, 6H), 3.50(m, 2H), 3.03~3.13(m, 4H), 2.60(m, 4H), 2.26(s, 2H), 1.40~1.89(m, 10H);13C NMRδ: 157.70, 156.90, 147.38, 144.75, 141.95, 132.37, 131.60, 127.20, 122.80, 122.60, 122.47, 121.96, 118.49, 114.39, 101.90, 77.22, 60.14, 56.73, 55.73, 42.68, 39.90, 27.72, 23.85, 15.29; FAB-MSm/z: 775.1{[M+H]+}。
2 结果与讨论
4a~5g均为已知化合物,其合成方法相似。目前文献[7~13]已报道的合成方法有两种:(1)以2或3为桥联基,分别与1a~1d在K2CO3和KOH存在下于甲苯中回流分水13 h制备。这种方法要用到毒性很强的甲苯作溶剂,而且反应温度高,反应时间长,产品需经柱层析纯化。(2)用NaH代替K2CO3和NaOH,以DMF代替甲苯作溶剂,这种方法的优点是反应条件温和,产率有所提高。但是作缚酸剂的NaH活性太强,而且NaH外层包有一层酯,反应之前必须用无水乙醚进行预处理,这样在操作过程中NaH很容易在空气中被氧化而着火,且乙醚在工业生产中也是很麻烦的溶剂,所以整个实验操作既繁琐又危险。
CaH2作缚酸剂活性适中,不需要像NaH那样作预处理,投料很方便,反应时间短,条件温和,粗产物用乙酸乙酯重结晶即可提纯。避免了复杂的柱层析。整个操作简单安全,适合工业化生产。其中4a,4b,5a和5b已经是工业化商品,其合成采用方法(1)。本文报道的合成路线(已经申请了一项国家专利)明显优于文献方法。
[1] Jacobsen E N, Marko I, Mungall W S,etal. Asymmetric dihydroxylation via ligand-accelerated catalysis[J].J Am Chem Soc,1988,110(6),1968-1970.
[2] Li G G, Hubert H A, Sharpless K B.N-halocarbamate salts lead to more effieient catalytic symmetric aminohydroxylation[J].J Angew Chem,Int Ed Engl,1996,35:2810-2813.
[3] Rudolph J, Sennhenn P C, Vlaar C P,etal. Smaller substituenton nitrogen faeilitate the osmium-catalyzed asymmetric amino-hydroxylation[J].J Angew Chem,Int Ed Engl,1996,35:451-454.
[4] Kolb H C, Vannieuwenhze M S, Sharpless K B. Catalytie asymmetric dihydroxylation[J].J Chem Rev,1994,94:2483-2547.
[5] Sharpless K B, Amberg W, Beller M,etal. New ligands double the scope of the catalytic asymmetric dihydroxylation of olefins[J].J Org Chem,1991,56(15):4585-4588.
[6] Sharpless K B, Amberg W, Bennani Y L,etal. The osmium-catalyzed asymmetric dihydroxylation:A new ligand class and a process improvement[J].J Org Chem,1992,57:2768-2771.
[7] Andersson M A, Epple R, Sharpless K B. A new approach to osmium-catalyzed asymmetric dihydroxylation and aminohydroxylation of olefins[J].Angew Chem Int Ed,2002,41(3):472-475.
[8] Irina Motorina, Cathleen M, Crudden Asymmetric dihydroxylatjon of olefins using cinchona alkaloids on highly ordered inorgnaic supports[J].J Organic Letters,2001,3(15):2325-2328.
[9] Choong Eui Song, Jung Woon Yang, Sang G Lee. Efficient and practical polymeric catalysts for heterogeneous asymmetric dihydroxylation of olefins[J].Tetrahedron:Asymmetry,1996,7(3):645-648.
[10] Jiang Ru, Kuang Y Q, Sun X L,etal. A improved catalytie system for recycling OsO4and chiral ligand in the asymmetric dihydroxlation of olefins[J].Tetrahedron Asymmetry,2004,15(4):743-746.
[11] Kuang Y Q, Zhang S Y, Jiang Ru,etal. A free ligand for the asymmetric dihydroxylation of olefins utilizing one-phase catalysis and two-phase separation[J]Tetrahedron lett,2002,43(20):3669-3671.
[12] Kuang Y Q, Zhang S Y, Wei L L. A simple and effeetive solublepolymer-bound ligand for the asymmetric dihydroxylation of olefins:DHQD-PHAL-OPEG-OMe[J].Tetrahedron Lett,2001,42:5925-5927.
[13] Braj B, Lohray E, Nandanan V B. Recent advances in the asymmetric dihydroxylation of alkenes[J].Tetrahedron Asymmetry,1992,3(11):1317-1349.
[14]4a:1H NMRδ: 8.66(d, 2H), 8.00(d, 2H), 7.40(d, 2H), 7.32(m, 4H), 7.23(d, 2H), 7.00(s, 2H), 6.75(s, 2H), 5.78(m, 2H), 4.95(m, 4H), 3.90(s, 6H), 3.37(m, 2H), 3.01(m, 4H), 2.60(m, 4H), 2.21(m, 2H), 1.50~1.81(m, 10H).4b:1H NMRδ: 8.65(d, 2H), 8.33(m, 2H), 7.98(d, 2H), 7.38(m, 4H), 7.27(s, 2H), 7.01(d, 2H), 3.92(s, 6H), 3.46(m, 2H), 3.11(m, 2H), 3.02(d, 1H), 2.99(d, 1H), 2.53(m, 2H), 2.31(d, 2H), 2.01(s, 1H), 1.79(d, 6H), 1.70(m, 2H), 1.40~1.28(m, 7H), 0.83(t, 6H).4c:1H NMRδ: 8.68(d, 2H), 8.00(d, 2H), 7.45(s, 2H), 7.38(m, 4H), 7.27(s, 2H), 7.00(s, 2H), 6.79(s, 2H), 5.80(m, 2H), 4.97(m, 4H), 3.92(s, 6H), 3.39(m, 2H), 3.08(m, 4H), 2.60(m, 4H), 2.26(m, 2H), 1.50~1.81(m, 10H);13C NMRδ: 160.74, 157.80, 147.27, 144.56, 144.18, 141.56, 131.47, 127.14, 121.99, 121.43, 114.54, 101.75, 77.26, 59.74, 56.42, 55.77, 42.56, 39.60, 27.57, 23.59, 16.96.4d:1H NMRδ: 8.67(d, 2H), 7.97(d, 2H), 7.42(s, 2H), 7.35(m, 4H), 7.26(d, 2H), 6.68(s, 2H), 6.73(s, 2H), 5.76(m, 2H), 4.90(m, 4H), 3.87(s, 6H), 3.30(m, 2H), 3.01(m, 4H), 2.43(m, 4H), 2.21(m, 2H), 1.45~1.73(m, 10H).4e:1H NMRδ: 8.66(d, 1H), 7.90(d, 1H), 7.42(s, 2H), 7.35(m, 2H), 7.27(s, 1H), 7.00(s, 1H), 6.75(s, 1H), 5.78(m,1H), 4.95(m, 2H), 3.90(s, 3H), 3.37(m, 1H), 3.01(m, 2H), 2.60(m, 2H), 2.21(m, 1H), 1.50~1.81(m, 4H).4g:1H NMRδ: 8.66(d, 1H), 7.90(d, 1H), 7.42(s, 2H), 7.35(m, 2H), 7.27(s, 1H), 7.29(d, 1H),4.00(s, 3H), 3.52~3.53(m, 1H), 2.74~2.95(m, 4H), 2.06(m, 1H), 1.77(s, 1H), 1.48~1.66(m, 6H), 0.91(t, 3H).5a:1H NMRδ: 8.65(d, 2H), 8.33(m, 2H), 7.98(d, 2H), 7.96(m, 2H), 7.56(d, 2H), 7.44(d, 2H), 7.36(d, 1H), 7.35(d,1H), 7.01(d, 2H), 3.90(s, 6H), 3.39(q, 2H), 2.82~2.60(m, 8H), 2.34(s, 1H), 2.20(s, 1H), 1.94(t, 2H), 1.68(s, 2H), 1.59~1.51(m, 4H), 1.46~1.39(m, 6H), 0.79(t, 6H);13C NMRδ: 157.58, 156.50, 147.38, 145.05, 144.75, 132.11, 131.56, 127.38, 122.80, 122.43, 121.78, 118.56, 102.09, 76.35, 60.27, 55.57, 50.89, 49.97, 37.46, 27.34, 26.29, 25.30, 23.20, 11.19.5b:1H NMRδ: 8.65(d, 2H), 8.33(m, 2H), 7.98(d, 2H), 7.94(m, 2H), 7.58(d, 2H), 7.43(d, 2H), 7.36(d, 1H), 7.35(d ,1H), 7.01(d, 2H), 3.92(s, 6H), 3.46(m, 2H), 3.11(m, 2H), 3.02(d, 1H), 2.99(d, 1H), 2.53(m, 2H), 2.31(d, 2H), 2.01(s, 1H), 1.79(d, 6H), 1.70(m, 2H), 1.40~1.28(m, 7H), 0.83(t, 6H);13C NMRδ: 157.62, 156.39, 147.35, 144.75, 144.73, 132.24, 131.51, 127.21, 122.79, 122.48, 121.97, 118.42, 101.99, 76.35, 60.01, 58.57, 55.64, 42.80, 37.46, 28.60, 27.68, 25.36, 23.54, 12.09.5d:1H NMRδ: 8.63(d, 2H), 8.36(m, 2H), 7.97(m, 4H), 7.53(d, 2H), 7.42~7.25(m, 4H), 7.03(m, 2H), 5.93(m, 2H), 4.99(m, 4H), 3.90(s, 6H), 3.41(d, 2H), 2.94(m, 8H), 2.23~2.05(m, 4H), 1.20~1.80(m, 10H);13C NMRδ: 157.66, 156.36, 147.32, 144.82, 140.29, 132.08, 131.53, 127.24, 122.98, 122.62, 122.42, 121.82, 118.25, 114.62, 102.02, 76.95, 60.08, 55.63, 49.82, 49.447, 39.63, 27.76, 26.46, 23.19; FAB-MSm/z: 775.6{[M+H]+}.5e:1H NMRδ: 8.67(d, 1H), 8.40(d, 1H), 8.20(d, 1H), 7.98~8.03(m, 2H), 7.63(s, 1H), 7.47(d, 1H), 7.28~7.38(m, 2H), 5.83~5.92(m, 1H), 5.00~5.06(m, 2H), 4.01(s, 3H), 3.61(t, 1H), 3.30(s, 1H), 3.09~3.16(m, 1H), 2.68~2.75(m, 2H), 2.33(s, 1H), 1.84~2.00(m, 4H), 1.61(s, 2H).5f:1H NMRδ: 8.64(d, 1H), 8.38(d, 1H), 8.21(d, 1H), 7.98~8.03(m, 2H), 7.62(s, 1H), 7.46(d, 1H), 7.28~7.39(m, 2H), 7.01(d, 1H), 3.90(s, 3H), 3.40(q, 1H), 2.82~2.62(m, 4H), 2.22(s, 1H), 1.94(t, 1H), 1.68(s, 1H), 1.59~1.1.51(m, 2H), 1.46~1.39(m, 3H), 0.79(t, 3H).5g:1H NMRδ: 8.67(d, 1H), 8.38~8.40(m, 1H), 8.15~8.17(m, 1H), 7.98(m, 2H), 7.96(d, 1H), 7.65(d, 1H), 7.48(d, 1H), 7.35(d, 1H), 7.29(d, 1H), 4.00(s, 3H), 3.52~3.53(m, 1H), 2.74~2.95(m, 4H), 2.06(m, 1H), 1.77(s, 1H), 1.48~1.66(m, 6H), 0.91(t, 3H).
