急性出血坏死性胰腺炎大鼠肠道黏膜屏障功能变化及己酮可可碱对其的保护作用
2011-11-21王庆刚雷若庆许志伟李红昌韩天权张圣道
王庆刚 雷若庆 许志伟 李红昌 韩天权 张圣道
·论著·
急性出血坏死性胰腺炎大鼠肠道黏膜屏障功能变化及己酮可可碱对其的保护作用
王庆刚 雷若庆 许志伟 李红昌 韩天权 张圣道
目的研究急性坏死性胰腺炎(ANP)大鼠肠道屏障功能改变及己酮可可碱(pentoxifylline,PTX)对肠道屏障的保护作用。方法54只SD雄性大鼠按数字表法随机分为ANP组、PTX组和假手术组。采用逆行胰胆管注射5%牛磺胆酸钠建立ANP模型。假手术组只翻动十二指肠。PTX组在制模后经阴茎静脉注射PTX 25 mg/kg体重。术后3、6、24 h分批处死大鼠。取血测淀粉酶、D-乳酸及TNF-α含量,取胰腺及末端回肠常规行病理学检查,免疫组化法检测回肠黏膜上皮紧密连接蛋白ZO-1的表达。结果建模后6 h,ANP组血清淀粉酶、TNF-α、D-乳酸含量分别为(9141±672)U/L、(347.96±79.47)pg/ml 和(10.21±1.08) mg/L,显著高于假手术组的(1723±57)U/L、(134.09±31.36)pg/ml和(4.33±0.49)mg/L(P值均<0.01) ;PTX组血淀粉酶、TNF-α、D-乳酸分别为(7965±318)U/L、(238.48±44.35)pg/ml和(8.75±1.28) mg/L,较ANP组显著降低,但仍显著高于假手术组(P<0.05或<0.01)。假手术组大鼠肠黏膜上皮ZO-1阳性率为(3.29±0.36)%;ANP组为(1.91±0.32)%,较假手术组明显减少(P<0.05);PTX组为(2.53±0.43)%,较假手术组减少,但较ANP组明显增加(P<0.05)。结论PTX可减轻ANP大鼠肠黏膜屏障功能的损伤,其机制可能是通过减少肠黏膜上皮ZO-1的降解。
胰腺炎,急性坏死性; 连接蛋白类; 己酮可可碱; 肠道屏障
重症急性胰腺炎(SAP)时肠道黏膜屏障在全身炎症反应综合征的形成过程中起到重要作用[1]。己酮可可碱(pentoxifylline,PTX)是一种非特异性磷酸二酯酶抑制剂,临床上作为血管扩张剂用于治疗周围血管疾病,同时用于改善微循环及缺血组织的氧供应[2]。近年研究发现,PTX能够调节炎症反应,降低肠道促炎症因子的合成[3-4]。本研究应用PTX干预急性坏死性胰腺炎大鼠,观察其对肠道黏膜屏障的保护作用。
材料与方法
一、动物分组及模型制作
54只健康雄性SD大鼠,体重200~250 g,SPF级,由瑞金医院实验动物中心提供。按数字表法随机分为ANP组、PTX组和假手术组,各18只。采用逆行胰胆管注射5%牛磺胆酸钠(Sigma公司)建立ANP模型。假手术组只翻动十二指肠后关腹。PTX组于制模后经阴茎静脉注射PTX 25 mg/kg体重,ANP组及假手术组注射等容积生理盐水。术后3、6、24 h分批处死每组大鼠各6只。腹主动脉取血,离心取血清,-80℃保存;取胰腺组织置4%多聚甲醛中固定;留取两段末端回肠,一段置-80℃保存,另一段于4%多聚甲醛中固定。
二、检测项目及方法
1.血淀粉酶、D-乳酸测定:淀粉酶由Beckmancoulter SYNCHRON CX4 PRO全自动生化仪检测。D-乳酸采用改良的酶学分光光度法测定[5]。
2.胰腺及末端回肠组织病理学检查:取甲醛固定的胰腺及回肠组织,常规石蜡包埋、切片、HE染色,由病理科医师阅片。
3.血清及回肠组织TNF-α浓度检测:回肠组织按每克加入5 ml PBS制成组织匀浆。TNF-α浓度采用ELISA法检测。
4.紧密连接蛋白ZO-1检测:取24 h处死大鼠的末端回肠组织,采用常规免疫组化SP法检测肠黏膜ZO-1蛋白表达。每张切片于光学显微镜下观察3个视野,应用Image-Pro Plus图片处理软件(Media Cybernetics公司)计算阳性表达百分率。
三、统计学方法
结 果
一、血淀粉酶、D-乳酸及TNF-α含量变化
ANP组大鼠血清淀粉酶、D-乳酸及TNF-α含量均较假手术组明显升高(P值均<0.01)。PTX组大鼠血淀粉酶、D-乳酸及TNF-α含量较ANP组明显降低,但仍较假手术组高(P<0.05或<0.01,表1)。
二、肠道组织TNF-α含量变化
ANP组大鼠肠道组织TNF-α含量较假手术组明显升高(P<0.01),而PTX组较ANP组明显下降,但仍较假手术组高(P值均<0.01,表1)。
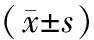
表1 各组大鼠血淀粉酶、D-乳酸、TNF-α含量及回肠组织TNF-α含量的变化
注:与假手术组比较,aP<0.01;与ANP组比较,bP<0.05,cP<0.01
三、胰腺及肠道组织的病理变化
假手术组大鼠胰腺组织结构完整;ANP组大鼠胰腺小叶结构模糊,呈片状坏死,间质内出血和大量炎细胞浸润;PTX组胰腺水肿,点片状坏死,较ANP组病理损伤明显减轻(图1上)。
假手术组大鼠回肠黏膜绒毛完整,排列整齐;ANP组回肠黏膜绒毛的完整性被破坏,部分固有层溃疡出血;PTX组回肠组织损伤较轻,仅表现为绒毛变短、变钝,绒毛结构完整(图1下)。

图1假手术组(左列)、ANP组(中列)、PTX组(右列)胰腺(上)及末端回肠(下)的病理学变化(HE ×100)
四、肠道黏膜上皮ZO-1蛋白表达的变化
肠道黏膜上皮ZO-1蛋白主要位于上皮细胞的边缘、细胞膜顶端,沿绒毛下方均匀连续分布,表达呈棕褐色。假手术组大鼠肠黏膜上皮染色较强,阳性表达率为(3.29±0.36)%;ANP组大鼠肠黏膜上皮染色较浅,分布不均,阳性表达率为(1.91±0.32)%(P<0.05);PTX组大鼠ZO-1阳性表达率为(2.53±0.43)%,较假手术组减少,但较ANP组有明显增加(P<0.05,图2)。
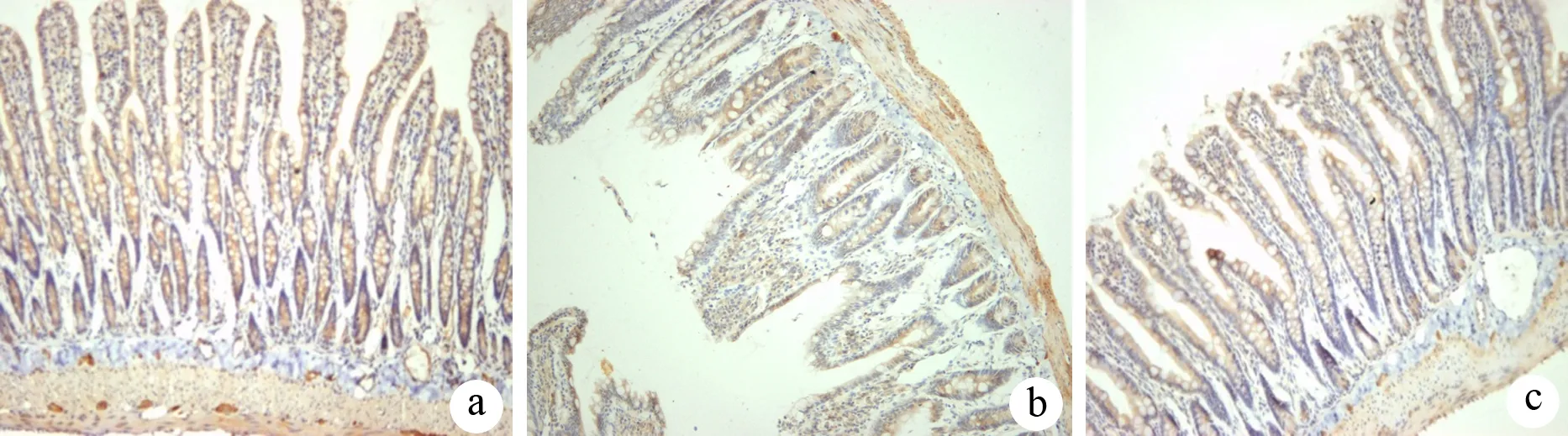
图2假手术组(a)、ANP组(b)、PTX组(c)肠道上皮紧密连接蛋白ZO-1的表达(免疫组化 ×100)
讨 论
肠道是人体的一个重要免疫器官,大约60%的T细胞位于小肠,同时肠道内也存在着大量的细菌及其产生的内毒素。生理情况下,机体凭借完整的肠黏膜屏障可以阻止这些细菌及内毒素进入体内。SAP早期往往伴有肠黏膜屏障损伤,上皮通透性增加,细菌及内毒素易位,从而加重急性胰腺炎病情[6]。
肠道黏膜上皮的通透性主要受肠道上皮细胞顶端的紧密连接控制,紧密连接蛋白ZO-1起着重要作用[7]。本实验结果显示,ANP大鼠肠黏膜上皮ZO-1蛋白表达较假手术组明显减少,而血清D-乳酸浓度较假手术组明显升高。说明ANP大鼠出现肠道屏障功能障碍。PTX组肠黏膜上皮ZO-1表达较ANP组明显改善,血D-乳酸浓度明显降低,说明PTX能够减轻ANP大鼠的肠黏膜损伤。
PTX能够调节炎症反应,降低促炎症因子的合成。TNF-α是一种促炎症介质,参与全身炎症反应综合征的发生,并且能够加速ZO-1的降解[8]。本结果显示,PTX组大鼠血清及肠道组织TNF-α浓度较ANP组显著降低,提示PTX降低血及肠道组织TNF-α浓度是其保护肠黏膜屏障的机制之一。
[1] Moore FA.The role of the gastrointestinal tract in postinjury multiple organfailure.Am J Surg,1999,178:449-453.
[2] 付玉民,王倩.己酮可可碱的药理研究及临床应用.医学综述,2001,7:509-510.
[3] Deree J,de Campos T,Shenvi E,et al.Hypertonic saline and pentoxifylline attenuates gut injury after hemorrhagic shock:the kinder,gentler resuscitation.J Trauma,2007,62:818-828.
[4] Coimbra R,Melbostad H,Loomis W,et al.Phosphodiesterase inhibition decreases nuclear factor-kappaB activation and shifts the cytokine response toward anti-inflammatory activity in acute endotoxemia.J Trauma,2005,59:575-582.
[5] 张家平,黄跃生,杨宗城.烧伤延迟复苏加重肠黏膜屏障功能损害的机制研究.世界华人消化杂志,2004,12:1329-1332.
[6] Ryan CM,Schmidt J,Lewandrowski K,et al.Gut macromolecular permeability in pancreatitis correlates with severity of disease in rats.Gastroenterology,1993,104:890-895.
[7] Fanning AS,Jameson BJ,Jesaitis LA,et al.The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton.J Biol Chem,1998,273:29745-29753.
[8] Poritz LS,Garver KI,Green C,et al.Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis.J Surg Res,2007,140:12-19.
2010-09-16)
(本文编辑:吕芳萍)
Changeofintestinalbarrierfunctioninacutenecrotizingpancreatitis(ANP)ratsandpentoxifylline′sprotectiveeffects
WANGQing-gang,LEIRuo-qing,XUZhi-wei,LIHong-chang,HANTian-quan,ZHANGSheng-dao.
DepartmentofSurgery,RuijinHospital,MedicineSchool,ShanghaiJiaotongUniversity,Shanghai20025,China
LEIRuo-qing,Email:ruoqinglei@yahoo.com.cn
ObjectiveTo investigate the change of intestinal barrier function and the protection of pentoxifylline (PTX) to intestinal barrier.MethodsFifty-four SD male rats were randomly divided into 3 groups, including sham operation group, ANP group, PTX group. ANP rat model were induced by retrograde injection of 5% sodium taurocholate into pancreatic and bile duct. Rats in sham operation group underwent operation without injection of taurocholate. After ANP induction, the rats in PTX group
PTX at a dose of 25 mg/kg weight via penis vein. The rats were sacrificed 3, 6, 24 h after operation, the serum levels of amylase, D-lactic acid, TNF-α were determined. The pancreas tissue and terminal ileum were harvested for pathological examination; ZO-1 levels of ileum epithelial tight junction were analyzed by immunohistochemistry.ResultsSix hours after induction, the serum levels of amylase, TNF-α, D-lactic acid in ANP group were (9141±672)U/L,(347.96±79.47)pg/ml and (10.21±1.08)mg/L, which were significantly higher than those in sham operation group [(1723±57)U/L, (134.09±31.36)pg/ml and (4.33±0.49)mg/L,P<0.01]. The serum levels of amylase, TNF-α, D-lactic acid in PTX group were (7965±318)U/L, (238.48±44.35)pg/ml and (8.75±1.28)mg/L, which were significantly lower than those in ANP group, but they were significantly higher than those in fsham group (P<0.05 or <0.01). The positive rate of ZO-1 was (3.29±0.36)% in sham operation group, and it was (1.91±0.32)% in ANP group, which was significantly lower than that in sham operation group (P<0.05); and the value was (2.53±0.43)% in PTX group, which was lower than that in sham group, but it was higher than that in ANP group (P<0.05).ConclusionsPTX may attenuate intestinal barrier function injury by decreasing the breakdown of intestinal ZO-1.
Pancreatitis,acute necrotizing; Connexins; Pentoxifylline; Enteval barrier
10.3760/cma.j.issn.1674-1935.2011.02.013
上海市重点学科——外科学(S30204)
200025 上海,上海交通大学医学院附属瑞金医院胆胰外科
雷若庆,Email:ruoqinglei@yahoo.com.cn
