环状二核铜配合物[Cu(nphth)(phen)(H2O)]2·2H2O的水热合成与晶体结构
2011-11-13姜秀榕温德才陈武华
姜秀榕 温德才 董 雁 陈武华
(龙岩学院化学与材料学院,龙岩 364000)
环状二核铜配合物[Cu(nphth)(phen)(H2O)]2·2H2O的水热合成与晶体结构
姜秀榕 温德才*董 雁 陈武华
(龙岩学院化学与材料学院,龙岩 364000)
在水热反应条件下,合成了一个新配合物,[Cu(nphth)(phen)(H2O)]2·2H2O(nphth=3-硝基邻苯二甲酸阴离子,phen=邻菲咯啉),经元素分析、红外、热重、单晶衍射表征了配合物。该配合物为单斜晶系,空间群 P21/n,结构参数:a=0.999 8(1)nm,b= 1.0445(1)nm,c=1.8206(1)nm,β=91.423(1)°,V=1.9007(2)nm3,Z=2,R=0.0394,wR=0.0965。结构分析表明,配合物为分立环状二核结构,并通过分子间氢键和π-π作用扩展为二维超分子网络结构。
铜配合物;3-硝基邻苯二甲酸;水热合成;晶体结构
0 Introduction
Self-assemblieshaveattracted considerableinterest in modern coordination chemistry and crystal engineering for the construction of discrete cyclic polynuclear complexes[1]and infinite polymers with potential applications[2].Many carboxylate ligands have been shown to be good building blocks in the preparing coordination polymerswith desired topologies owing to their rich coordination modes[3].o-Phthalic acid is a versatile ligand for designing new supramolecular constructions.Up to now,Numerous complexeswith ophthalate ligands were found to display diverse structure types[4],but only a few 3d-series transition metal complexes with 3-nitrophthalate ligand are reported[5].Herein,we report the hydrothermalsynthesis and crystalstructure ofanovelcyclic discretebinuclear copper(Ⅲ) complex, [Cu(nphth)(phen)(H2O)]2·2H2O(nphth=3-nitrophthalate,phen=1,10-phenanthroline), withmixed ligandsof3-nitrophthalateand phen.
1 Experimental
1.1 Reagentand apparatus
All of the chemicals were obtained from commercial sources and were used without further purification.Elemental analyseswere conducted on a Perkin-Elmer 2400 CHN elemental analyzer.The IR spectra were recorded on a Nicolet360 FTIR spectrometerwith KBr pellets in the 4 000~400 cm-1region.Thermogravimetric analysiswas obtained on NETZSCH STA 449C thermogravimetric analyzer,carried outunder N2with a heating rate ofat10℃·min-1.
1.2 Synthesisof[Cu(nphth)(phen)(H 2O)]2·2H2O
Amixture of Cu(NO3)2·6H2O(0.061 g,0.2mmol), phen(0.016 g,0.2mmol),3-nitrophthalic acid(0.042 g, 0.2 mmol)and distilled water(10mL)was put into a Teflon-lined autoclave(20mL)and then heated at403 K for 72 h.Blue block-like crystals of the title complex in 50%yield based on Cu.Anal.Calcd.for C40H30N6O16Cu(%):C,49.13;H,3.09;N,8.60.Found(%):C, 49.33;H,3.19;N,8.50.
1.3 Crystalstructure determ ination
A blue block-like single crystalwith dimension of 0.23mm×0.21mm×0.20mm for the title complex was used for X-ray diffraction analysis.Data collection was carried out at 293 K on a Rigaku RAXIS-RAPID Weissengberg IP diffractometer with graphitemonochrocmated Mo Kαradiation(λ=0.071073 nm)inωscan mode.A total of 12 743 reflections and 4 100 unique oneswere collected in the range of 2.35°≤θ≤27.00° with Rint=0.0302,ofwhich3 135 reflectionswith I>2σ(I) were considered as observed and used in the succeeding structural calculations.The structure was solved by direct method and difference Fourier syntheses.The aromatic H atoms were positioned geometrically and were included in the refinement in the riding-model approximation (C-H=0.093 nm and Uiso(H)=1.2Ueq(C)).The water H atomswere found in a difference Fouriermap and were refined with distance restraints of O-H=0.085(1)nm and Uiso(H)=1.2Ueq(O).All calculations were carried out with SHELX 97 program[6].
The Crystal belongs to monoclinic system,the space group is P21/n,with the crystalcellparameters a= 0.999 8(1)nm,b=1.044 5(1)nm,c=1.820 6(1)nm,β= 91.423(1)°,and V=1.900 7(2)nm3,Mr=977.78,Dc= 1.708 g·cm-3,μ=1.208mm-1,F(000)=996,Z=2,R= 0.039 4,wR=0.096 5,S=1.031,Δ/σ=0.000,Δρmax=783 e·nm-3andΔρmin=-441 e·nm-3.
CCDC:801643.
2 Resultsand discussions
2.1 IR spectra
The IR spectrum of the title complex reveals two strong bonds of the carboxylic groups at 1 590 and 1379 cm-1for the asymmetric vibrationsand symmetric vibrations,respectively.The difference between the asymmetric and symmetric stretching vibrations(Δν= νas(COO-)-νs(COO-))is 211 cm-1,suggesting that the carboxyl group coordinates to copper(Ⅲ) with monodentatemode[7].A strong peak at1524 cm-1isassigned to νas(aromatic NO2)and the other strong peak at1350 cm-1isassigned toνs(aromatic NO2).Thewide peaksat about 3 451,3 066 cm-1are assigned to OH vibration, suggesting the existence of the crystal and coordinated watermoleculesand H-bonding in the complex.
2.2 Crystalstructure
The molecular structure of the title complex is shown in Fig.1.The selected bond distancesand angles of the title complex are given in Table1.

Fig.1 Cyclic binuclear structure of the title complex with 30%probability ellipsoid
The Cu1 atom in title complex adopts a squarepyramidal N2O3environment,with the basal plane defined by atoms N1 and N2 from phen,atom O2 from one 3-nitrophthalate ligand,and O7 from a H2O,O4A atom from the other 3-nitrophthalate ligand occupies the apical position with a Cu1-O4A distance of 0.2230(1)nm.Two Cu(Ⅲ)centers are linked by carboxylate groups from two phth2-bridges,showing a good centro-symmetry in the whole molecule.Thus,a discrete cyclic binuclear structure is constructed by two Cu(Ⅲ)atoms,two phen and two phth bridges,the core of the binuclear structure being an fourteen-membered ringwith Cu…Cu distanceof0.5227(1)nm.
The title complex has rich hydrogen bonds formed by the crystal and coordinated water molecules and uncoordinated oxygen atoms of carboxylate groups. Detailed data are given in Table 2.Then adjacent binuclear structure units are connected together by the hydrogen bonds to 1D chain along the a axis.There are π-πinteractions between the aromatic rings of phen of the neighboring chain with a centroid-centroid distance between neighbouring aromatic ringsof 0.3595(1)nm, generating an extended two-dimensional architecture (Fig.2).

Table 1 Selected bond lengths(nm)and angles(°)for the title comp lex
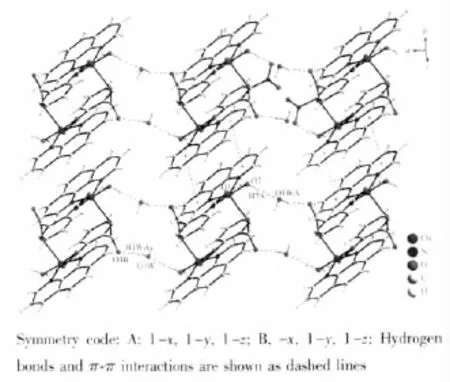
Fig.2 Extended 2D structure in the title complex
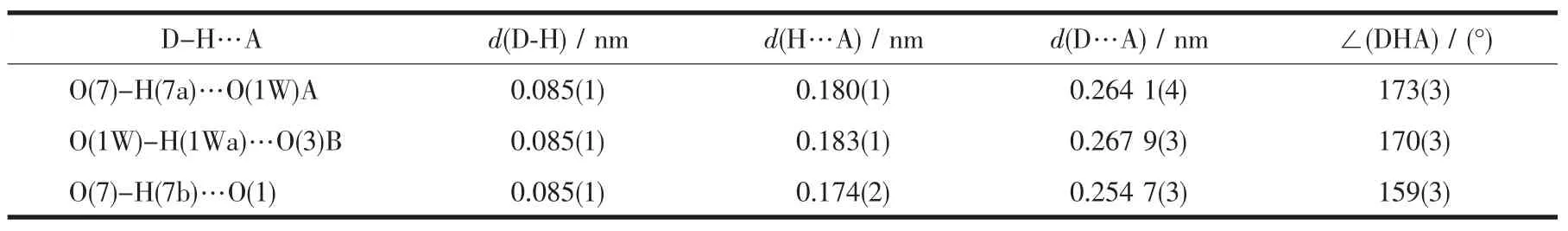
Table 2 Hydrogen bond geometry for the comp lex
2.3 Thermogravimetric analysis
The thermogravimetric analysis of the title complex was performed under N2atomsphere.The weight loss of7.40% (calcd.7.36%)between 110 and 156℃ corresponds to the loss of the crystal and coordinated water molecules.The main framework began to collapseatabove156℃.
In summary,a novel discrete cyclic binuclear complex[Cu(nphth)(phen)(H2O)]2·2H2O bridged by two 3-nitrophthalate was synthesized.A two-dimensional supramolecularnetwork isconstructed through hydrogen bondsandπ-πinteractions.
[1](a)Leininger S,Olenyuk B,Stang P J.Chem.Rev.,2000,100: 853-908
(b)Liu SX,Lin S,Lin B Z,etal.Angew.Chem.Int.Ed.Engl., 2001,40:1084-1087
(c)Zhang JP,Lin Y Y,Huang X C,et al.Chem.Commun., 2005:1258-1260
(d)Wang C F,Gao EQ,He Z,et al.Chem.Commun.,2004: 720-721
(e)Hu T L,Li JR,Liu CS,et al.Inorg.Chem.,2006,45:162-173
[2](a)Moulton B,Zaworotko M J.Chem.Rev.,2001,101:1629-1658
(b)EddaoudiM,MolerDB,LiH,etal.Acc.Chem.Res.,2001, 34:319-330
[3](a)Rosi N L,Eddaoudi JK M,Chen B,et al.J.Am.Chem. Soc.,2005,127:1504-1518
(b)Ye B H,Tong M L,Chen X M.Cood.Chem.Rev.,2005, 249:545-565
(c)LU Zhen-Da(鲁振达),YAO Jing(姚景),LIN Jian-Guo(林建国),etal.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2008,24(8):1335-1342
(d)Zhao B,Chen XY,Cheng P,etal.J.Am.Chem.Soc.,2004, 126:15394-15395
(e)LiX J,Wang X Y,Gao S,etal.Inorg.Chem.,2006,45:1508 -1516
(f)LIQiang(李强),ZHENG Fa-Kun(郑发鲲),CAILi-Zhen(蔡丽珍),etal.Chinese J.Struct.Chem.(Jiegou Huaxue),2005, 24(3):354-358
(g)Wen D C,Liu S X,Ribas J.Polyhedron,2007,26:3849-3856
[4](a)Yang JM,Zhou ZH,Zhang H,et al.Inorg.Chim.Acta, 2005,358:1841-1849
(b)Ma CB,WangW G,Zhang X F,etal.Eur.J.Inorg.Chem., 2004:3522-3532
(c)Wang X L,Qin C,Wang E B,et al.J.Mol.Struct.,2005, 737:49-54
(d)Ye CH,Sun H L,Wang X Y,etal.J.Chem.Cryst.,2005, 35:381-384
(e)Kato M,Sah A K,Tanase T,et al.Inorg.Chem.,2006,45: 6646-6660
(f)Sun LP,Niu SY,Jin J,etal.Inorg.Chem.Commun.,2006, 9:679-682
(g)Tian L,Chen L,Shen SH,etal.J.Coord.Chem.,2007,60: 683-689
[5](a)Deng Y H,Liu J,Wu B,et al.Eur.J.Inorg.Chem.,2008: 1712-1718
(b)Song Y S,Yan B,Chen Z X.Appl.Organometal.Chem., 2007,21:150-155
(c)Shen XQ,Qiao H B,LiZ J,etal.Inorg.Chim.Acta,2006, 359:642-648
[6]Sheldrick GM.SHELX-97,Program for RefinementofCrystal Structure,University ofGöttingen,Germany,1997.
[7]Zheng Y,Xu DM,Liu SX.Inorg.Chim.Acta,1999,294:163-169
Hydrothermal Synthesis and Crystal Structure of a Cyclic Binuclear Copper Com plex[Cu(nphth)(phen)(H2O)]2·2H2O
JIANG Xiu-Rong WEN De-Cai*DONG Yan CHENWu-Hua
(College of Chemistry and Materials Science,Longyan University,Longyan,Fujian 364000,China)
A novel complex[Cu(nphth)(phen)(H2O)]2·2H2O(nphth=3-nitrophthalate,phen=1,10-phenanthroline) was synthesized by hydrothermal reaction and characterized by elementalanalysis,IR,TGA and X-ray single crystal diffraction.The title complex crystallizes inmonoclinic with space group P21/n,a=0.999 8(1)nm,b=1.044 5(1)nm, c=1.8206(1)nm,β=91.423(1)°,V=1.9007(2)nm3,Z=2,R=0.039 4,wR=0.096 5.The structure analysis shows that the complex exhibits a discrete cyclic binuclear structure.The 2D supramolecular network is constructed through hydrogen bondsandπ-πinteraction.CCDC:801643.
copper complex;3-nitrophthalic acid;hydrothermal synthesis;crystal structure
O614.121
A
1001-4861(2011)02-0393-04
2010-07-27。收修改稿日期:2010-10-18。
福建省教育厅资助项目(No.JB09221)。
*通讯联系人。E-mail:wendecai1227@yahoo.com.cn
