一氧化氮抑制缺氧诱导因子α亚基表达对大鼠缺氧性肺动脉高压的作用*
2011-10-24陈云荣戴爱国胡瑞成
陈云荣, 戴爱国, 胡瑞成
(湖南省老年医院-湖南省老年医学研究所呼吸疾病研究室, 湖南 长沙 410016)
一氧化氮抑制缺氧诱导因子α亚基表达对大鼠缺氧性肺动脉高压的作用*
陈云荣, 戴爱国△, 胡瑞成
(湖南省老年医院-湖南省老年医学研究所呼吸疾病研究室, 湖南 长沙 410016)
目的研究缺氧诱导因子α亚基(HIF-α)在一氧化氮(NO)抑制缺氧性肺动脉高压形成中的作用。方法32只成年雄性SD大鼠随机分为4组:常氧对照组(C组)、单纯缺氧组(H组)、缺氧加左旋精氨酸组(L-Arg组)、缺氧加左旋精氨酸甲酯组(L-NAME组)。3个缺氧组常压缺氧(10%)21 d并每天1次腹腔注射相应药物。测定各组大鼠平均肺动脉压(mPAP)、右室肥大指数(RVHI)、血管形态学指标;原位杂交和RT-PCR检测HIF-α、诱导型一氧化氮合酶(iNOS) mRNA的表达,免疫组化和Western blotting检测HIF-α、iNOS蛋白质的表达。结果3个缺氧组肺组织NO浓度较C组降低,且L-Arg组肺组织NO浓度高于H组,L-NAME组肺组织NO浓度低于H组。3个缺氧组mPAP、RVHI、血管壁面积与血管面积比值(WA%)、肺动脉中膜厚度(PAMT)都较对照组增高(P<0.05)。L-Arg组mPAP和PAMT较H组低(P<0.05),RVHI和WA%与H组比较差异无显著。L-NAME组mPAP、RVHI、WA%和PAMT均较H组高(P<0.05)。3个缺氧组HIF-1α和HIF-3α mRNA表达较C组增高(P<0.05),3个缺氧组间HIF-1α mRNA表达差异无显著,而L-NAME组HIF-3α mRNA表达高于L-Arg组(P<0.05),其它组间无显著差异。L-NAME组HIF-2α mRNA高于C组,其它组之间差异无显著。3个缺氧组HIF-1α、HIF-2α和HIF-3α蛋白质表达较C组均增高(P<0.05),且L-Arg组表达低于H组(P<0.05),L-NAME组高于H组(P<0.05)。直线相关分析表明,大鼠肺组织NO浓度与HIF-α蛋白质、iNOS mRNA及蛋白质表达水平、mPAP、RVHI、WA%和PAMT呈负相关(均P<0.05)。结论在缺氧性肺动脉高压大鼠模型中NO主要在转录后下调HIF-α的表达,NO下调HIF-α的表达可能是其抑制缺氧性肺动脉高压形成的重要机制。
缺氧诱导因子; 一氧化氮; 高血压,肺性; 缺氧
缺氧诱导因子(HIF)是由α、β亚基组成的异二聚体,其β亚基为结构亚基,对氧稳定;α亚基(HIF-α)是功能亚基,对氧敏感。HIF-α家族包括3个成员HIF-1α,HIF-2α、HIF-3α,在缺氧性肺动脉高压形成过程中有重要作用,我们最近研究表明,缺氧时肺小血管HIF-α蛋白质表达增高,在缺氧大鼠和慢性阻塞性肺疾病患者的HPH发病中起着重要作用[1-3]。一氧化氮(nitric oxide,NO) 可通过调节血管张力和血管平滑肌细胞的增殖而影响HPH的发生、发展。最近研究表明:缺氧时NO可降低HIF-α蛋白质的稳定性,阻止缺氧诱导的HIF-α的蓄积。HIF-α可调节诱导型一氧化氮合酶(inducible nitric oxide synthase,iNOS)的表达从而影响NO的合成。 NO和HIF-α在缺氧性肺动脉高压形成过程中的相互调控值得研究。本实验通过观察大鼠HPH模型中左旋精氨酸(L-arginine,L-Arg)和左旋硝基精氨酸甲酯(Nω-nitro-L-arginine methyl ester,L-NAME)对肺组织、肺小血管HIF-α及表达的影响,探讨NO是否通过调节HIF-α表达抑制缺氧性肺动脉高压形成及NO和HIF-α在缺氧性肺动脉高压形成过程中的相互调控,为HPH的防治提供新的理论依据。
材 料 和 方 法
1动物模型复制及分组
32只清洁级成年雄性健康SD大鼠 (湖南中医学院实验动物中心提供),体重(270±30)g,随机数字表法分为4组,每组8只:常氧对照组,3个缺氧组。常氧对照组(C组)每天腹腔注射生理盐水1 mL。3个缺氧组分别为:单纯缺氧组(H组),每天缺氧前腹腔注射生理盐水1 mL;缺氧加L-Arg组(L-Arg组),每天缺氧前腹腔注射L-Arg(Sigma)500 mg/kg;缺氧加L-NAME组(L-NAME组),每天缺氧前腹腔注射L-NAME(Sigma)5 mg/kg。3个缺氧组按本研究室传统方法[2]每天(10.0±0.5)%氧浓度常压缺氧8 h。
2平均肺动脉压(meanpulmonaryarterialpressure,mPAP)测定及右室肥厚指数(rightventriclehypertrophyindex,RVHI)的测定
按照我室的传统方法[2]大鼠经1%戊巴比妥钠(40 mg/kg) 腹腔麻醉后,经右颈外静脉插入微导管,通过压力传感器接上Medlab生物信号采集系统(南京美易科技有限公司) 测大鼠mPAP。经以上检测后将大鼠处死,取出心脏置4 %多聚甲醛固定48 h,分别称取右室(RV) 和左室+室间隔(LV+S) 的湿重,按RVHI=RV/ (LV+S) 计算RVHI。
3肺小血管形态学分析
大鼠左侧肺组织以4%多聚甲醛固定,石蜡包埋,切片,厚度4 μm,苏木精-伊红(HE) 染色,每只大鼠选3张肺组织切片,每张切片以单盲法选取断面积较圆的直径100 μm左右的肺细小动脉5支,用病理图像分析软件(PIPS22020型,重庆天海医疗设备有限公司)测定肺动脉管壁面积/管总面积(ratio of vascular wall area to total vascular area,WA%)和肺动脉中膜厚度(pulmonary artery media thickness,PAMT),作为肺小血管重塑指标。
4NO浓度测定

5肺小血管壁HIF-α和iNOS的原位杂交
寡核苷酸探针,地高辛标记的多相寡核苷酸探针(武汉博士德生物工程有限公司提供) 探针序列。HIF-1α:(1)5’-TTATG AGCTT GCTCA TCAGT TGCCA CTTCC-3’; (2)5’-CTCAG TTTGA ACTAA CTGGA CACAG TGTGT-3’; (3)5’-GGCCG CTCAA TTTAT GAATA TTATC ATGCT-3’。HIF-2α:(1) 5’-CGAAC ACATA AACTC CTGTC TTCAG TGTGC-3’;(2)5’-ATCCG AGAGA ACCTG ACACT CAAAA CTGGC-3’; (3)5’-GGGCA AGTGA GAGTC TACAA CAACT GCCCC-3’.HIF-3α:(1)5’-CGCAT GCACC GCCTC TGCGC TGCAG GGGAG-3’;(2)5’-ACATG GCTTA CCTGT CGGAA AATGT CAGCA-3’;(3)5’-ATATG AGGGC CTACA AGCCC CCTGC ACAGA-3’。iNOS:(1)5’-GTGGC GTAAA GTATG TGTCT GCAGA TATGC TGGAA-3’;(2)5’-GAAGC CATGA CCTTC CGCAT TAGCA GAGAA GCAAA-3’; (3)5’-TCTTC GGGCTTCAGG TTATT GATCC AAGTG CTGCA-3’。参照说明书及Hu等[4]杂交步骤,二氨基联苯胺(DAB) 显色棕黄色为阳性结果(主要在胞浆)。结果观察: 每只大鼠选2张结构完整的切片,每张切片以单盲法选3支直径100 μm左右的肺小动脉,图像分析软件(PIPS22020型,重庆天海医疗设备有限公司)检测肺细小动脉管壁平均吸光度(A) 值作为管壁HIF-1α、HIF-2α、HIF-3α和iNOS表达相对含量。
6RT-PCR测肺组织中HIF-α和iNOSmRNA的转录
取右肺组织0.1 g,按说明书用Trizol试剂(上海生工)提取总RNA, 逆转录后进行PCR,扩增引物由上海生物公司合成。PCR 反应条件: 94 ℃预变性5 min,94 ℃变性30 s,适宜温度退火30 s,72 ℃延伸60 s,反应适当循环,最后72 ℃延伸10 min。RT-PCR产物经1.5 % 的琼脂糖凝胶(含0.5 mg/L 溴化乙啶) 电泳,凝胶图像分析系统(上海Tanon Gis-2010)对扩增产物条带吸光度半定量,计算待测条带的吸光度与内参照β-actin的吸光度比值。引物序列、退火温度、产物长度、循环数见表1。
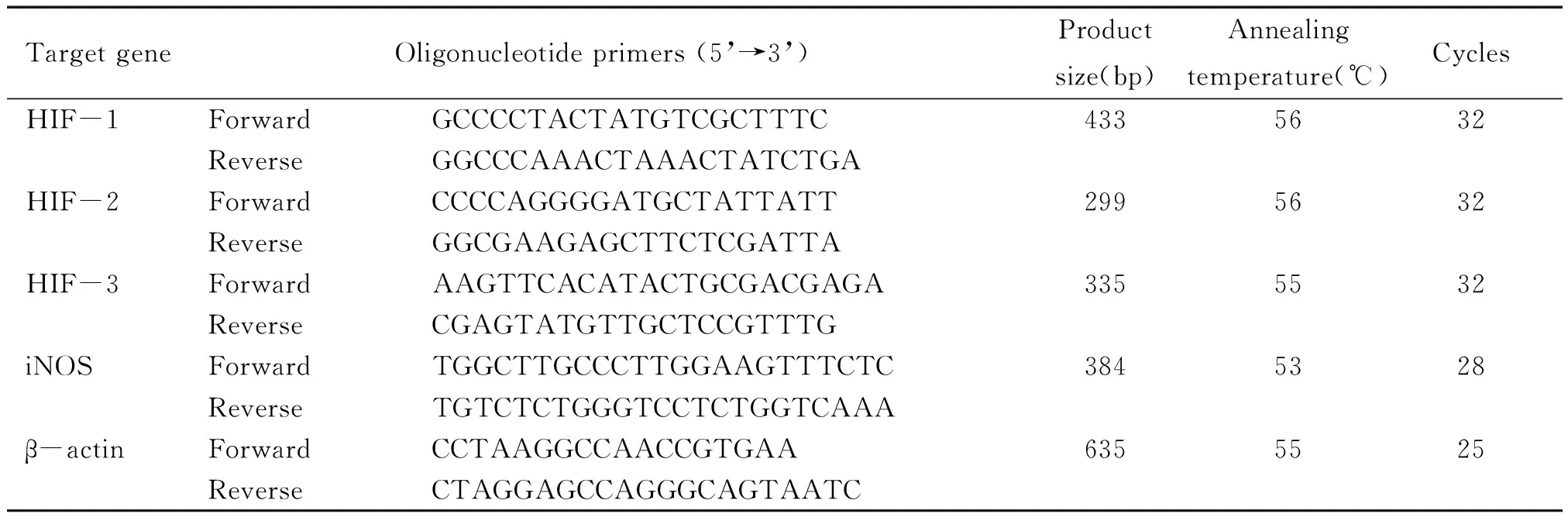
表1 各目的基因的引物序列、退火温度、产物长度、循环数
7肺小血管壁HIF-α和iNOS免疫组织化学
Ⅰ抗:HIF-1α,HIF-2α、HIF-3α、iNOS均为兔抗大鼠多克隆抗体(Santa Cruz),1∶200稀释。Ⅱ抗SABC测检试剂盒(购自武汉博士德公司)。操作步骤参照李启芳等[2]的方法, DAB显色阳性结果(主要在胞浆) 显棕黄色。结果观察:肺小动脉血管选择以及管壁HIF-α和iNOS的蛋白表达相对含量检测同原位杂交。
8Westernblotting检测肺组织中HIF-α和iNOS蛋白质表达水平
取大鼠右侧肺组织0.1 g,剪碎加入1 mL改良RIPA裂解液[50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl、0.5%脱氧胆酸钠、1 mmol/L EDTA、1% NP-40、0.1 mg/L PMSF和2 mg/L亮抑素],冰浴条件下组织匀浆; 4 ℃ 10 000×g离心,弃除沉淀,上清液即为肺组织总蛋白。Bradford法测定上清液总蛋白浓度,样品分装、贮存于-80 ℃中备用。每孔加入等量蛋白质样品,7.5%十二烷基硫酸钠-丙烯酰胺凝胶200伏恒压电泳后,转印至硝酸纤维膜。5%脱脂奶粉室温封闭2 h后,与1∶1 000稀释的Ⅰ抗(Santa Cruz) 4 ℃温育过夜。封闭液漂洗后加入1∶2 000稀释的Ⅱ抗(Santa Cruz),室温下摇床杂交1 h。漂洗后增强化学发光法发光,X线胶片显影,图像分析系统(上海Tanon Gis-2010)对条带吸光度半定量,计算待测条带的吸光度与内参照β-actin的吸光度比值。
9统计学处理
结 果
1NO抑制缺氧性肺血管重塑和肺动脉压升高
3个缺氧组肺组织匀浆NO浓度均降低,L-Arg组肺组织匀浆NO浓度高于H组,L-NAME组低于H组,见表2;3个缺氧组mPAP、RVHI都增高,并发生血管重塑。L-Arg组mPAP、RVHI较H组低(P<0.05),血管重塑不如H组明显,L-NAME组mPAP、RVHI较H组高(P<0.05),血管重塑比H组更明显,见表2。
表2各组大鼠肺组织NO浓度及mPAP、RVHI(%)、WA%


GroupNO(mol/gprotein)mPAP(mmHg)RVHI(%)WA(%)PAMT(μm)Control12.49±2.4816.9±2.523.8±3.834.8±5.58.4±1.3L-Arg7.95±1.25*24.8±3.2*26.8±2.5*55.6±5.6*16.5±2.2*Hypoxia5.47±0.91*△30.4±4.5*△28.9±1.9*61.2±4.9*19.8±3.1*△L-NAME2.08±0.36*△#35.1±3.9*△#32.0±1.6*△#69.8±7.0*△#23.3±2.9*△#
*P<0.05vscontrol;△P<0.05vsL-Arg;#P<0.05vshypoxia.
2NO抑制缺氧诱导的缺氧诱导因子升高
2.1不同组HIF-α mRNA表达变化 原位杂交示肺小动脉HIF-1α mRNA在对照组呈阳性表达,缺氧组HIF-1α mRNA表达增高,呈较强阳性或强阳性表达,但H组、L-Arg组、L-NAME组3个缺氧组间HIF-1α mRNA表达差异无显著,见图2;RT-PCR示肺组织HIF-1α mRNA表达变化规律与肺小动脉相同,见表3、图1。原位杂交示各组大鼠肺小动脉HIF-2α mRNA呈阳性表达,L-NAME组HIF-2α mRNA表达略高于C组,见表3、图3。其它组间表达差异无显著;RT-PCR示4组大鼠肺组织HIF-2α mRNA均有明显表达,但各组间表达无明显差异,见图1。原位杂交示HIF-3α mRNA在对照组呈弱阳性表达,3个缺氧组HIF-3α mRNA均呈阳性或较强阳性表达,见表3、图4,且L-NAME组HIF-3α mRNA表达高于L-Arg组(P<0.05),L-Arg组、L-NAME组与H组间比较差异均无显著,RT-PCR示对照组HIF-3α mRNA表达较弱,3个缺氧组HIF-3α mRNA表达均较C组增高,见图1。

Figure 1. Reverse transcription-polymerase chain reaction analysis of hypoxia-inducible factor (HIF)-α subunits (HIF-1α, HIF-2α and HIF-3α)and iNOS mRNA C:control; H, hypoxia for 21 d; L-Arg: hypoxia for 21 d with administration of L-Arg; L-NAME: hypoxia for 21d with administration of L-NAME. The amplification of β-actin was used as a control.
图1RT-PCR检测各组大鼠肺组织HIF-1α、HIF-2α、HIF-3α和iNOSmRNA的表达

Figure 2.Insituhybridization analysis of hypoxia-inducible factor (HIF)-1α mRNA expression in rat pulmonary arteries after exposure to hypoxia for 0 d (A) (control), 21 d(B), or hypoxia for 21 d with administration of L-Arg(C) and L-NAME(D)(DAB,×400). HIF-1α mRNA was positively stained in control (A), and increased a little after exposure to hypoxia(B) (P<0.05), but no significant differences were observed among the three hypoxia groups(B, C and D).
图2肺小血管壁HIF-1αmRNA表达

Figure 3.Insituhybridization analysis of hypoxia-inducible factor (HIF)-2α mRNA expression in rat pulmonary arteries after exposure to hypoxia for 0 d (A) (control), 21 d(B), or hypoxia for 21 d with administration of L-Arg(C) and L-NAME(D) (DAB, ×400). HIF-2α mRNA was positively stained in control(A),and increased a little after exposure to hypoxia with administration of L-NAME(D) (P<0.05), but no significant differences were observed among A,B and C).
图3肺小血管壁HIF-2αmRNA表达

Figure 4.Insituhybridization analysis of hypoxia-inducible factor (HIF)-3α mRNA expression in rat pulmonary arteries after exposure to hypoxia for 0 d (A) (control), 21 d(B), or hypoxia for 21 d with administration of L-Arg(C) and L-NAME(D)(DAB, ×400). HIF-3α mRNA was poor positively stained in control (A), and increased significantly after exposure to hypoxia.
图4肺小血管壁HIF-3αmRNA表达
2.2HIF-α protein表达变化 Western blotting示HIF-1α、HIF-2α蛋白在C组大鼠肺组织中有表达,HIF-3α在C组大鼠肺组织中呈较弱表达,缺氧组大鼠HIF-1α、HIF-2α、HIF-3α表达均明显增高,且它们在L-Arg组大鼠肺组织表达水平低于C组,而L-NAME组大鼠,肺组织表达水平高于C组,见图5。免疫组化示HIF-1α蛋白在对照组肺小血管表达不明显,在肺小血管中L-Arg组呈弱阳性表达,H组、L-NAME组呈阳性表达,且L-NAME组表达更明显,见表3、图6。HIF-2α蛋白在对照组肺小血管呈弱阳性表达,L-Arg组呈阳性表达,H组、L-NAME组呈强阳性表达,L-NAME组表达更明显,见表3、图7。HIF-3α蛋白在对照组肺小血管未见明显表达(支气管上皮呈弱阳性表达),L-Arg组呈阳性表达,H组、L-NAME组呈较强阳性表达,且L-NAME组表达更明显,见表3、图8。
3NO抑制缺氧诱导的iNOS表达升高
原位杂交示及免疫组化示对照组大鼠肺小血管iNOS mRNA和蛋白质呈阳性表达,见表3、图9、10, H组iNOS mRNA和蛋白质呈强阳性表达。H组iNOS与L-NAME组、L-Arg组比较差别无显著,但L-NAME组iNOS与L-Arg组比较差异显著。RT-PCR结果与原位杂交结果一致,见图5。

Figure 5. Western blotting analysis of hypoxia-inducible factor (HIF)-α subunits (HIF-1α, HIF-2α and HIF-3α) and iNOS protein levels in rat lung tissues during normoxia (control) and hypoxia for 21 d with administration of L-Arg and L-NAME. C: control; H: hypoxia for 21 d; L-Arg: hypoxia for 21d with administration of L-Arg; L-NAME: hypoxia for 21 d with administration of L-NAME. The amplification of β-actin was used as a control.
图5各组大鼠肺组织HIF-1α、HIF-2α、HIF-3α和iNOS蛋白质的表达
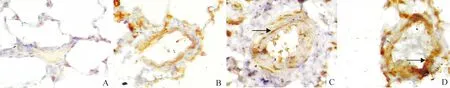
Figure 6. Immunohistochemistry analysis of hypoxia-inducible factor (HIF)-1α protein expression in rat pulmonary arteries after exposure to hypoxia for 0 d (A) (control), 21 d(B), or hypoxia for 21 d with administration of L-Arg(C) and L-NAME(D)(DAB,×400). HIF-1α protein was induced in all three hypoxia groups, and it was significantly lower in L-Arg group, but significantly higher in L-NAME group than that in H group.
图6肺小血管壁HIF-1α蛋白质表达

Figure 7. Immunohistochemistry analysis of hypoxia-inducible factor (HIF)-2α protein expression in rat pulmonary arteries after exposure to hypoxia for 0 d (A) (control), 21 d(B), or hypoxia for 21 d with administration of L-Arg(C) and L-NAME(D) (DAB,×400). HIF-2α protein were induced in all three hypoxia groups, and it was significantly lower in L-Arg group, but significantly higher in L-NAME group than that in H group.
图7肺小血管壁HIF-2α蛋白质表达

Figure 8. Immunohistochemistry analysis of hypoxia-inducible factor (HIF)-3α protein expression in rat pulmonary arteries after exposure to hypoxia for 0 d (A) (control), 21d(B), or hypoxia for 21d with administration of L-Arg(C) and L-NAME(D) (DAB,×400). HIF-3α protein were induced in all three hypoxia groups, and it was significantly lower in L-Arg group, but significantly higher in L-NAME group than that in H group.
图8肺小血管壁HIF-3α蛋白质表达
表3NO对大鼠肺小动脉HIF-1α、HIF-2α、HIF-3α和iNOSmRNA和蛋白表达的影响


GroupHIF-1αmRNAHIF-2αmRNAHIF-3αmRNAHIF-1αproteinHIF-2αproteinHIF-3αproteiniNOSmRNAiNOSproteinControl0.143±0.0180.130±0.0180.090±0.0050.082±0.0110.096±0.0070.050±0.0070.125±0.0170.130±0.020 L-Arg0.173±0.018*0.153±0.0160.220±0.020*0.112±0.012*0.184±0.019*0.141±0.017*0.232±0.029*0.223±0.030*Hypoxia0.172±0.020*0.152±0.0180.239±0.028*0.145±0.017*△0.239±0.028*△0.182±0.029*△0.248±0.041*0.244±0.025*L-NAME0.185±0.018*0.159±0.016*0.257±0.029*△0.205±0.021*△#0.294±0.028*△#0.233±0.025*△#0.283±0.032*△#0.272±0.032*△#
*P<0.05vscontrol;△P<0.05vsL-Arg;#P<0.05vshypoxia.

Figure 9.Insituhybridization analysis of iNOS mRNA expression in rat pulmonary arteries after exposure to hypoxia for 0 d (A)(control), 21 d(B), or hypoxia for 21 d with administration of L-Arg(C) and L-NAME(D) (DAB,×400). iNOS mRNA was increased in all three hypoxia groups, and was significantly higher in L-NAME group than that in H group, but no significant difference was observed between L-Arg group and H group.
图9肺小血管壁iNOSmRNA表达

Figure 10. Immunohistochemistry analysis of iNOS mRNA expression in rat pulmonary arteries after exposure to hypoxia for 0 d (A)(control), 21 d(B), or hypoxia for 21 d with administration of L-Arg(C) and L-NAME(D) (DAB,×400). iNOS protein was increased in all three hypoxia groups, and was significantly higher in L-NAME group than than in H group, but no significant difference was observed between L-Arg group and H group.
图10肺小血管壁iNOS蛋白质表达
4NO浓度与HIF-α蛋白质、iNOS表达及非小血管重塑指标的相关性
直线相关分析表明,大鼠肺组织NO浓度与HIF-1α(r=-0.85)、HIF-2α(r=-0.89)、HIF-3α(r=-0.88)蛋白质、iNOS mRNA(r=-0.82)、iNOS蛋白质(r=-0.84)表达水平及PAMT(r=-0.87)、RVHI(r=-0.74)、mPAP(r=-0.83)、WA%(r=-0.87)呈负相关(均P<0.01)。
讨 论
早期研究发现NO和L-Arg可通过抑制内皮素1(ET-1)、血管内皮生长因子(VEGF)等的表达[5],抑制HPH和HPSR的发生发展。HIF对常氧和缺氧状态下介导生理和病理过程起非常关键的作用,Manalo等[6]认为肺动脉内皮细胞可能有5%的基因受HIF-1调控。我们研究发现HPH发展的不同阶段HIF-α亚基基因表达在肺血管壁的动态变化有明显差异[7],缺氧时HIF-1α可调控其靶基因iNOS、血红素氧合酶1等表达,参与缺氧大鼠和慢性阻塞性肺疾病(COPD)患者缺氧性肺血管重塑(HPSR)的形成。我们研究还发现有丝分裂原激活蛋白激酶(MAPKs)、磷脂酰肌醇3激酶(PI3K)通路调控HIFs,参与在缺氧大鼠和COPD患者HPSR的形成[8]。低浓度NO可减少缺氧[9,10]及去铁胺[11]诱导的HIF-1α及其靶基因在培养细胞内的表达增高。本实验中L-Arg组大鼠HIF-α表达均降低,L-NAME组大鼠HIF-α表达均增高,且HIF-1α、HIF-2α、HIF-3α蛋白质水平与肺组织NO浓度均呈负相关,提示缺氧性肺动脉高压大鼠模型中NO可能通过调节HIF-α的表达,进而调节其靶基因的表达。
我们先前研究表明,缺氧时肺组织HIF-1α在肺组织蓄积,可诱导iNOS表达增高,使NO合成增加[4]。本实验中iNOS表达变化的趋势与HIF-α一致,3个缺氧组(L-Arg组、单纯缺氧组、L-NAME组)iNOS水平均增高,且L-Arg组表达低于H组,L-NAME组高于H组。iNOS的表达与HIF-α呈正相关,而与NO呈负相关,提示NO可能通过抑制HIF-α的表达,降低iNOS的表达水平,使NO合成减少。
缺氧对HIF-α的表达调控主要通过影响HIF-α蛋白质的稳定性,我们研究发现大鼠缺氧性肺动脉高压发生发展过程中HIF-1α、HIF-2α mRNA仅轻度升高,而蛋白质表达水平明显升高,说明其表达主要发生在蛋白质水平。低浓度NO可减少缺氧及去铁胺诱导的HIF-1α在培养细胞内的蓄积,而对HIF-1 α mRNA水平无影响,说明NO主要通过对HIF-α的蛋白质稳定性调节HIF-α的蛋白质水平[8-10]。本实验中3个缺氧组(H组、L-Arg组、和L-NAME组)肺组织和肺小血管HIF-α蛋白质水平均增高,且L-Arg组表达低于H组,L-NAME组高于H组,而3个缺氧组间mRNA表达水平差异无显著,提示NO对HIF-α表达的调控发生在蛋白质水平。
我们研究发现HIF脯氨酸羟化酶(HIF prolyl hydroxylase,PHD)表达和活性的改变是导致HIF-α差异性表达的重要原因,NO降低HIF-α蛋白质稳定性的作用可能与cGMP-蛋白激酶途径无关,而与PHD活性有关[12]。Hagen等[9]发现这是由于线粒体细胞色素C氧化酶对O2的Km值比PHD低,NO抑制细胞色素氧化酶,氧分子重新分布,细胞内氧浓度增加,PHD活性增强,HIF-1α的降解增加,其它线粒体呼吸抑制剂也有相同作用。Callapina等[10]发现NO可使细胞内活性氧簇(ROS)形成增加,增强PHD活性,清除ROS后NO的作用减弱。Kozhukhar等[13]认为NO损伤线粒体及线粒体呼吸链,线粒体内2-酮戊二酸、亚铁离子释放入胞质增加PHD的活性。本实验中NO增加HIF-α降解的机制是否通过增强PHD活性尚需进一步研究。尚有研究发现常氧时NO可以增加HIF-1α的蛋白质稳定性[14,15],但本实验中NO增加了HIF-α的降解,导致HIF-α蛋白质水平下降及其靶基因表达的下调。
[1] 李启芳, 戴爱国, 徐 平. 慢性阻塞性肺疾病患者肺小血管低氧诱导因子-α的表达[J]. 中华内科杂志, 2006, 45(2): 136-139.
[2] 李启芳, 戴爱国. 大鼠缺氧性肺动脉高压过程中缺氧诱导因子1α和血红素氧合酶1的变化[J]. 中国病理生理杂志, 2005, 21(7): 1260-1264.
[3] 李炽观, 戴爱国, 严鹏科. 低氧诱导因子1在低氧致肺动脉平滑肌细胞增殖中的作用[J]. 中国病理生理杂志, 2007,23(7): 1301-1305.
[4] Hu R, Dai A, Tan S. Hypoxia-inducible factor 1 alpha upregulates the expression of inducible nitric oxide synthase gene in pulmonary arteries of hypoxic rat[J]. Chin Med J(Engl), 2002, 115(12): 1833-1837.
[5] Blumberg FC, Wolf K, Sandner P, et al. The NO donor molsidomine reduces endothelin-1 gene expression in chronic hypoxic rat lungs[J]. Am J Physiol Lung Cell Mol Physiol, 2001, 280(2): L258-L263.
[6] Manalo DJ,Rowan A,Lavoie T,et al.Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1[J].Blood,2005,105(2):659-669.
[7] Chen YR, Dai AG, Hu RC, et al. Differential and reciprocal regulation between hypoxia-inducible factor-alpha subunits and their prolyl hydroxylases in pulmonary arteries of rat with hypoxia-induced hypertension[J]. Acta Biochim Biophys Sin, 2006, 38(6): 423-434.
[8] 孔春初, 戴爱国. 磷酸肌醇3-激酶调控缺氧诱导因子1α对大鼠缺氧性肺动脉高压的作用[J]. 中国病理生理杂志, 2006, 22(11): 2132-2137.
[9] Hagen T, Taylor CT, Lam F, et al. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α[J]. Science, 2003, 302(5652): 1975-1978.
[10]Callapina M, Zhou J, Schmid T, et al. NO restores HIF-1alpha hydroxylation during hypoxia: Role of reactive oxygen species[J]. Free Radic Biol Med, 2005, 39(7): 925-936.
[11]Callapina M, Zhou J, Schnitzer S, et al. Nitric oxide reverses desferrioxamine- and hypoxia-evoked HIF-1α accumulation-implications for prolyl hydroxylase activity and iron[J]. Exp Cell Res, 2005, 306(1): 274-284.
[12]Wang F, Sekine H, Kikuchi Y, et al. HIF-1α-prolyl hydroxylase: molecular target of nitric oxide in the hypoxic signal transduction pathway[J]. Biochem Biophys Res Commun, 2002, 295(3): 657-662.
[13]Kozhukhar AV, Yasinska IM, Sumbayev VV. Nitric oxide inhibits HIF-1α protein accumulation under hypoxic conditions: implication of 2-oxoglutarate and iron[J]. Biochimie, 2006, 88(5): 411-418.
[14]Metzen E, Zhou J, Jelkmann W, et al. Nitric oxide impairs normoxic degradation of HIF-1α by inhibition of prolyl hydroxylases[J]. Mol Biol Cell, 2003, 14(8): 3470-3481.
[15]Quintero M, Brennan PA, Thomas GJ, et al. Nitric oxide is a factor in the stabilization of hypoxia-inducible factor-1α in cancer: role of free radical formation[J]. Cancer Res, 2006, 66(2): 770-774.
Inhibitionofhypoxia-induciblefactorαexpressionbynitricoxideinratswithhypoxicpulmonaryhypertension
CHEN Yun-rong, DAI Ai-guo, HU Rui-cheng
(DepartmentofRespiratoryMedicine,HunanInstituteofGerontology,HunanProvinceGeriatricHospital,Changsha410001,China.E-mail:daiaiguo2003@163.com)
AIM: To investigate the effect of nitric oxide (NO) on the expression of hypoxia-inducible factor α(HIF-α) in rats with hypoxic pulmonary hypertension.METHODSL-arginine was used as the NO donor. Male SD rats (n=32) were randomly divided into 4 groups: 1 group of normoxia (C group) and 3 groups of hypoxia. The rats in hypoxia groups were exposed to 10% oxygen merely (H group), and with administration of L-arginine (L-Arg group) or nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME group). Mean pulmonary arterial pressure (mPAP), pulmonary artery morphometry and right ventricle hypertrophy index (RVHI) were measured. RT-PCR andinsituhybridization were used to detect the mRNA expression. Immunohistochemistry and Western blotting were adopted to determine the expression of the proteins.RESULTSThe NO concentrations of the lung tissues in 3 hypoxic groups were lower than that in C group. In H group, the NO concentration was lower than that in L-Arg group, bwt higher than that in L-NAME group. mPAP, RVHI, ratio of vascular wall area to total vascular area(WA%) and pulmonary artery media thickness(PAMT) were higher in hypoxic groups than those in C group. mPAP and PAMT in L-Arg group were significantly lower than those in H group (P<0.05), but those in L-NAME group were signficantly higher (P<0.05) WA% and RVHI in L-NAME group were significantly higher than those in H group(P<0.05), but no significant difference was observed between H group and L-Arg group. Compared with C group, the mRNA expression of HIF-1α and HIF-3α increased in hypoxia groups (P<0.05).For HIF-1α mRNA, no significant difference was observed among the 3 hypoxia groups, while the alteration of HIF-3α mRNA was more obvious in L-NAME group than that in H group (P<0.05). HIF-2α mRNA was higher in L-NAME group than that in H group (P<0.05), but no significant difference was observed among other groups. The proteins of HIF-α were induced in all 3 hypoxia groups (P<0.05), and they were significantly lower in L-Arg group (P<0.05)but significantly higher in L-NAME group (P<0.05) than that in H groups. Linear correlation analysis showed the negative correlations between NO concentration and the HIF-α protein, iNOS mRNA and protein, mPAP, RVHI, WA%, PAMT.CONCLUSIONNitric oxide may down-regulate HIF-α expression via posttranscriptional modification. The NO-mediated increase in HIF-α expression may be potentially involved in the inhibition of developing hypoxic pulmonary hypertension.
Hypoxia-inducible factor; Nitric oxide; Hypertension, pulmonary; Hypoxia
R363
A
10.3969/j.issn.1000-4718.2011.01.003
1000-4718(2011)01-0014-08
2010-06-20
2010-10-23
国家自然科学基金资助项目 (No.30570815);湖南省自然科学基金资助项目(No.07JJ3035);湖南省卫生厅课题资助项目(No.B2006-178)
△通讯作者 Tel:0731-84762793; E-mail: daiaiguo2003@163.com
