Pemetrexed in Previously Treated Non-small Cell Lung Cancer Patients with Poor Performance Status
2011-09-11SunYoungJUNGSuJinYOOJiYoungSHINJiWonPARKJeongEunLEEHeeSunPARKJuOckKIMSunYoungKIM
Sun Young JUNG, Su Jin YOO, Ji Young SHIN, Ji Won PARK, Jeong Eun LEE, Hee Sun PARK, Ju Ock KIM,Sun Young KIM
Department of Internal Medicine, Chungnam National University Hospital, Daejon, Korea
Correspondence to: SunYoung KIM, Department of Internal Medicine, Chungnam National University, Daejon, Korea
E-mail: sykim@cnu.ac.kr
Abstract Background and objective Pemetrexed have been approved for the treatment of patients affiected by advanced non-small cell lung cancner (NSCLC) in progression after fi rst-line chemotherapy. We evaluated the activity and feasibility of pemetrexed in previously treated NSCLC.
Key words Pemetrexed; Lung neoplasms; Poor performance
Introduction
Lung cancer is the leading cause of cancer-related deaths in Korea and worldwide[1,2]. More than 80% of patients with lung cancer have non-small cell lung cancer(NSCLC) histology[3]. Additionally, most NSCLC patients are diagnosed with locally advanced or metastatic disease.For advanced disease, systemic chemotherapy is the standard therapy. The standard fi rst-line therapy for patients with NSCLC is platinum-based doublet combination chemotherapy, which offers a modest survival advantage[4].As second-line chemotherapeutic agents, various drugs such as docetaxel, pemetrexed, and the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) erlotinib and gef i tinib have been used. Among them, pemetrexed is a multi-target antifolate second-line chemotherapeutic agent[5]that shows effiects comparable to docetaxel.
Presently, numerous NSCLC patients receive higher than third-line chemotherapy; however, a guideline for chemotherapy has not been established for these patients.Pemetrexed is less toxic than other therapeutics, and thus could be used safely in the elderly and in cases with poor Eastern Cooperative Oncology Group performance status(ECOG PS)[7,8]. Thus, pemetrexed could be used for later than third-line chemotherapy. However, until now, few studies have investigated the efficacy of pemetrexed in later than third-line chemotherapy with poor PS.
In the present study, the efficacy of pemetrexed in later than third-line chemotherapy in NSCLC patients with poor PS was examined retrospectively.
Patients and methods
Patients
The study population consisted of 56 patients who received pemetrexed because of progression after treatment at Chungnam National University Hospital between April 2007 and March 2009. Clinicopathologic data and follow-up information were retrieved from patient medical records. All patients had histological or cytological conf i rmation of locally advanced or metastatic NSCLC, stage IIIb or IV [tumor node metastasis (TNM) staging], and poor ECOG PS (≥2).
Treatment
All patients received 500 mg/m2pemetrexed as a 10 min intravenous infusion on day 1 and every 21 days thereafter.Folic acid supplementation (1,000 mg) was orally administered daily beginning approximately 1-2 weeks before the first dose of pemetrexed and continued until 3 weeks after treatment discontinuation. A 1,000 mg vitamin B12injection was administered intramuscularly approximately 1-2 weeks before the first dose of pemetrexed and was repeated approximately every 9 weeks until 3 weeks after therapy discontinuation.Dexamethasone was administered twice daily on the day before,the day of and the day after each dose of pemetrexed. Treatment was continued until disease progression, unacceptable toxicity,or the patient or physician decided to discontinue therapy.
Response
Tumor responses were assessed with computed tomography every two or three cycles to evaluate treatment responses for documentation of disease progression. Additionally, for cases with headache, nausea, vomiting, and other neurological symptoms (which together could indicate brain metastasis),systemic metastasis was evaluated by performing brain magnetic resonance imaging. For cases suspected to have bone metastasis, systemic metastasis was evaluated by bone scan or positron emission tomography-CT. Objective tumor responses were based on Response Evaluation Criteria in Solid Tumors(RECIST) criteria[9].
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software (version 17.0;SPSS, Inc., Chicago, IL, USA). The survival period was from the day of the first administration of pemetrexed to death or the date that the follow-up observation was terminated.The progression-free survival (PFS) encompassed the time from the first cycle of pemetrexed therapy to documented progression or death from any cause until the date of the last follow-up visit for patients who were still alive and whose cancer had not progressed. For the analysis of survival period and PFS, the Kaplan-Meier method was used, and P-values were obtained based on the Log-rank test.
For comparison of the characteristics of the patient groups, treatment response rate, and control of disease, the chi-square test was used. For analysis of factors mediating effiects on survival, the Logistic regression model was used.Statistical signif i cance was deemed to be P<0.05.
Results
Patient characteristics and distribution
Among 56 patients, 11 (19.6%) were treated with pemetrexed as second-line therapy, and 45 (80.4%) were administered pemetrexed as later than third-line therapy.The study group included 38 males (67.9%) and 18 females(32.1%). The age distribution was 43-81 years: 15 patients(26.8%) were >70 years, and 41 patients (73.2%) were<70 years. For patients >70 years, 63.6% were in secondline treatment, whereas 17.8% were in later than third-line treatment. All 56 patients had an ECOG PS ≥2. In total, 32 patients had non-squamous cell carcinoma (adenocarcinoma,n=29; indeterminate non-small-cell carcinoma, n=3), and 24(42.9%) patients had squamous cell carcinoma. Regarding disease stage, 16 patients exhibited less than stage IIIb, and 40 exhibited stage IV disease. Almost all patients had been treated with a platinum-based chemotherapy as first-line treatment: 38 underwent a gemcitabine-platinum regimen,10 received a taxane-platinum regimen, and 5 were treated with an irinotecan-platinum regimen. The best response to fi rst-line therapy was as follows: partial response (PR) in 25 patients (44.6% of evaluable patients), stable disease (SD)in 15 patients (26.8%), and progressive disease (PD) in 16 patients (28.6%) (Tab 1).
Response
Among 56 cases, complete remission was achieved in one case (1.8%), partial remission was achieved in eight cases (14.3%), no change occurred in 14 cases (25.0%), and progressive lesions were found in 33 cases (58.9%). The treatment response rate was 16.1%, and the disease control rate was 41.1% (Tab 2). The median survival was 6.11 months, and the median PFS was 2.17 months.
Subgroup analysis
The non-squamous cell cancer group included 18 cases(56.2%), and in comparison with the 5 cases in the squamous cell cancer group (20.8%), the disease control rate in the former group was significantly higher (P=0.018). Similarly,the treatment response rate for the non-squamous epithelial cancer group was 25.0% (8 cases), which was significantly higher than the 4.1% (1 case) for the squamous epithelial cancer group (P=0.045). By multivariate analysis, only histology showed persistent predictive relevance (Tab 3).
Efficacy of third- or further-line pemetrexed therapy
Pemetrexed was administered as part of second-line(19.6% of the patients), third-line (23.2% of the patients),fourth-line (35.7% of patients), and later than fifth-line chemotherapy (21.4% of the patients). In 80.4% of patients,pemetrexed was administered as later than third-line chemotherapy. When second-line was compared with later than third-line therapy, the median PFS was higher in later than third-line therapy (1.50 months vs 2.25 months; P=0.017,1). The median survival of second-line chemotherapy was not significantly different from that in later than third-line chemotherapy (11.18 months vs 11.46 months; P=0.922,5).
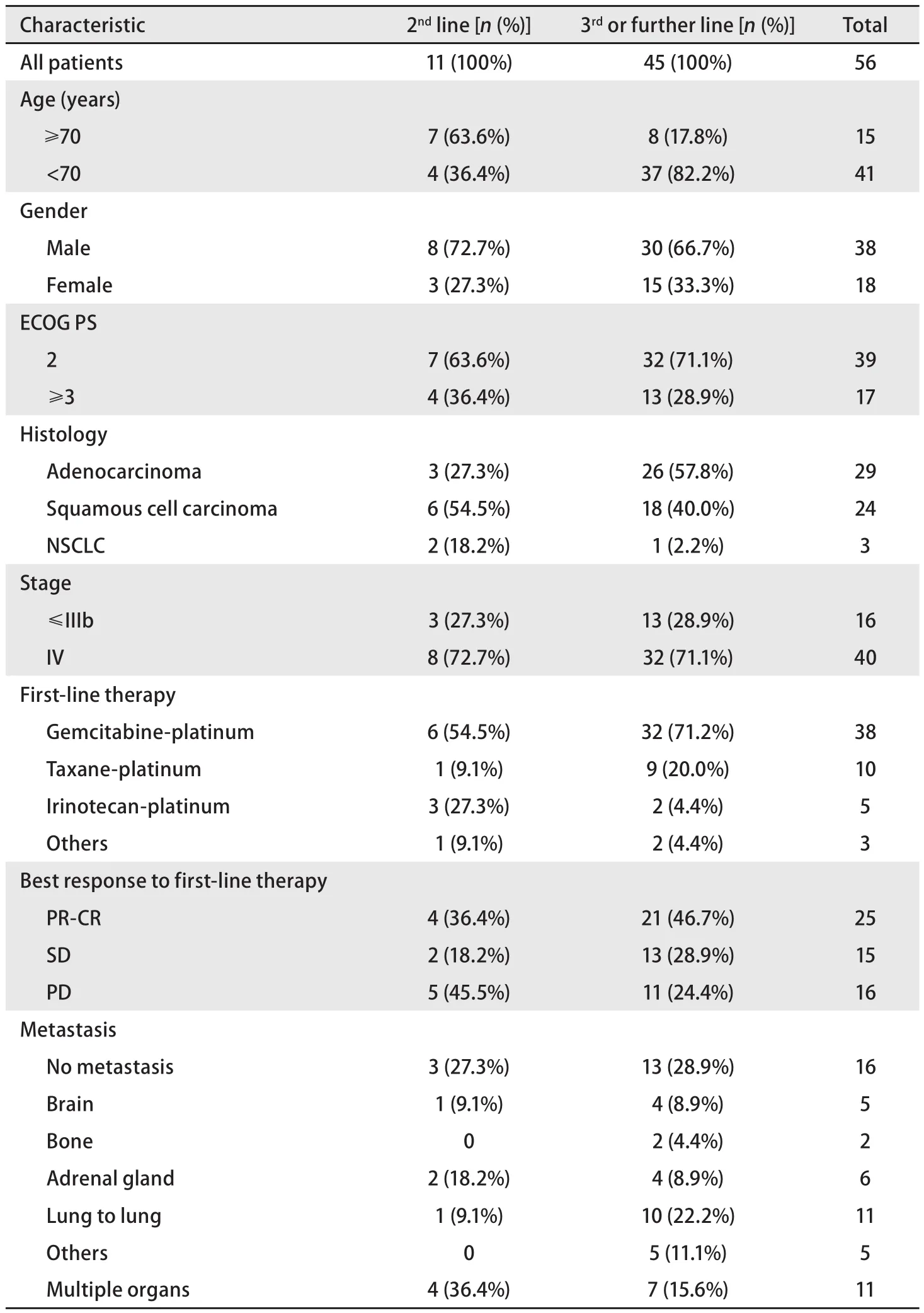
Tab 1 Patients characteristics (n=56)
Toxicity
The toxicity prof i les of all patients are summarized in Tab 4. Anemia was the most frequent adverse event (28.4%),followed by nausea (23%). Other adverse events were less frequent. Grade 3-4 hematologic toxicity occurred in 8.8% of cases. None of the patients required dose modif i cations due to toxicity.
Discussion
Presently, fi rst-line chemotherapy for progressive NSCLC consists of combination platinum-based chemotherapies[10].For cases with disease recurrence or progressive disease after fi rst-line therapy, docetaxel, pemetrexed, or an EGFRTKI (erlotinib or gefitinib) may be used. Among them,the median survival of docetaxel has been shown to be signif i cantly longer than optimal palliative therapy, and thusa survival benef i t has been conf i rmed[11].

Tab 2 Best response data for each line pemetrexed therapy
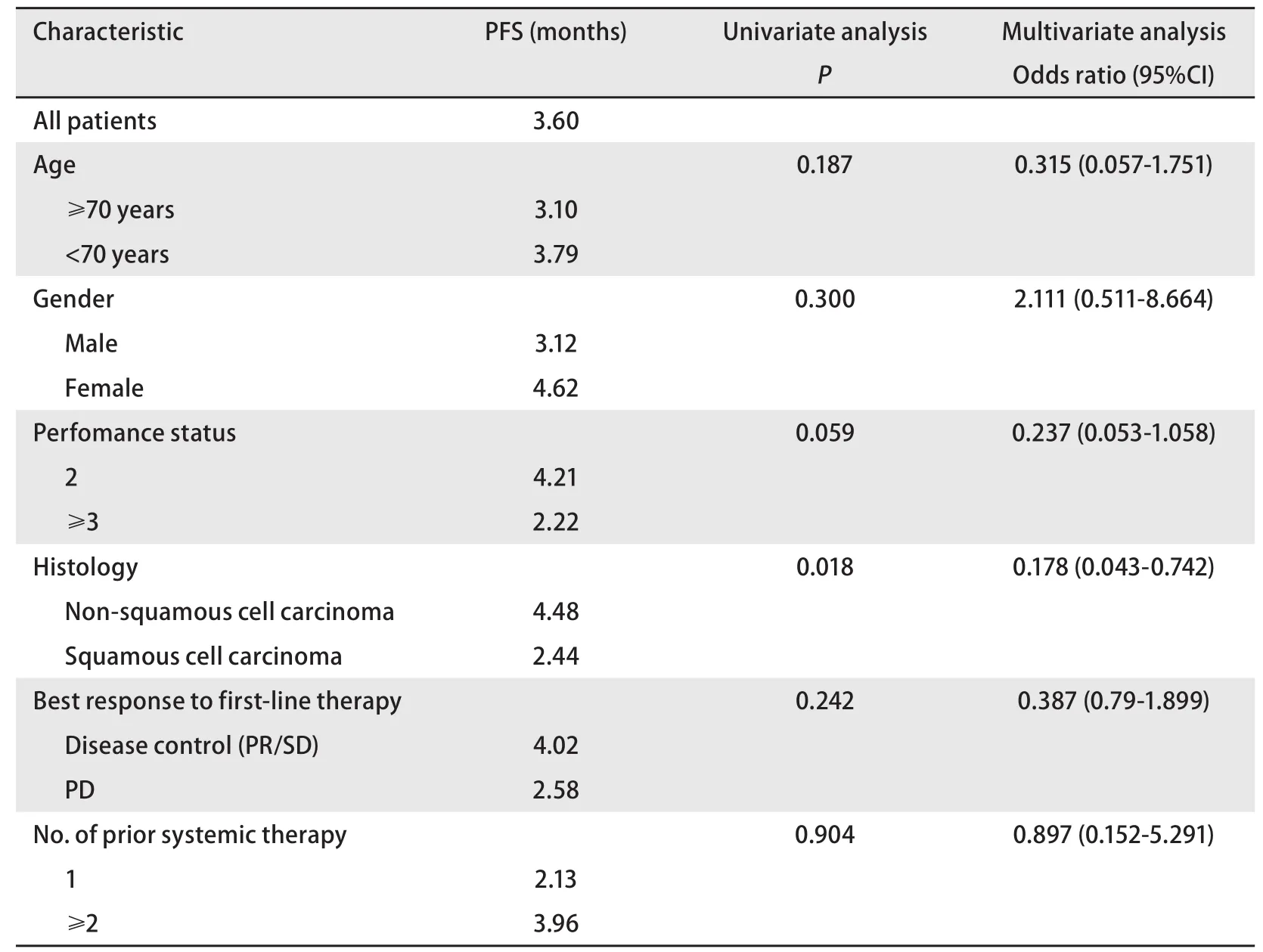
Tab 3 Univariate and multivariate analyses for progression-free survival
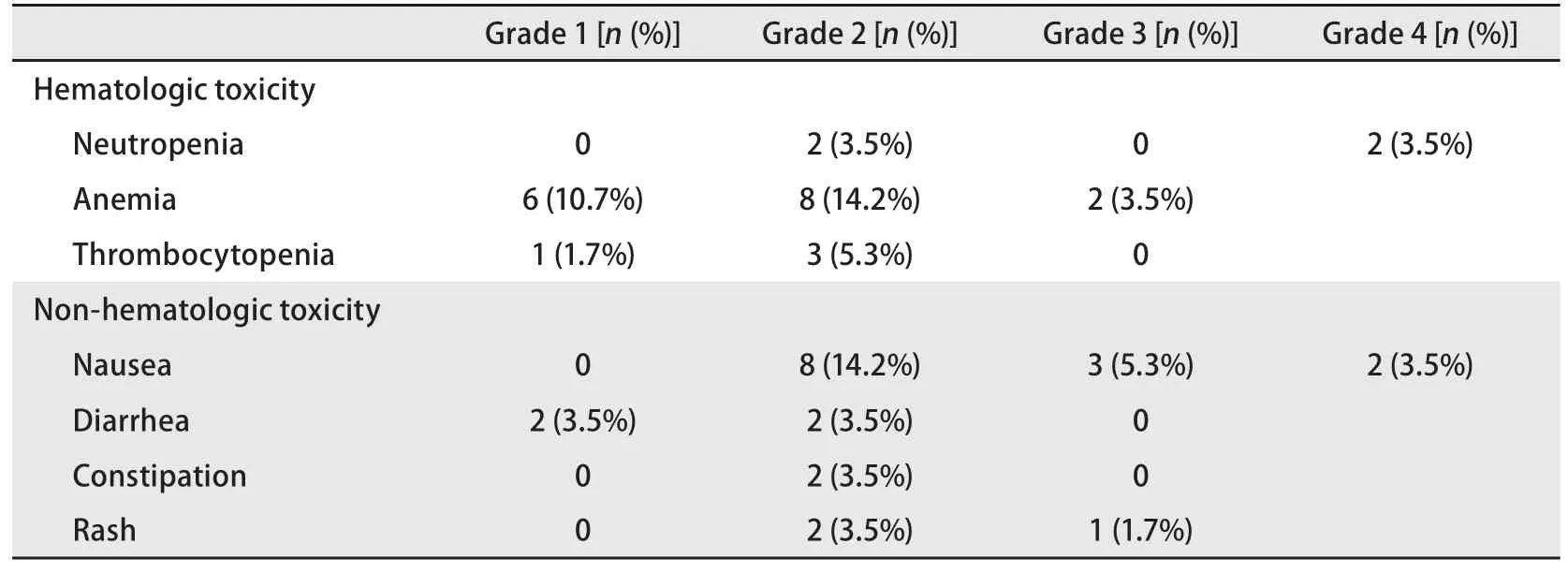
Tab 4 Toxicity profiles
Compared with docetaxel, pemetrexed has demonstrated a similar median survival, and thus its efficacy is accepted.Importantly, its side effects were revealed to be safer than those from docetaxel[6,12].
In our study, pemetrexed was used as later than secondline chemotherapy for NSCLC treatment. The treatment response rate was 16.1%, and the disease control rate was 41.1%. The median survival was 6.11 months, and the median PFS was 2.17 months. Compared with the squamous cell cancer group, the non-squamous cell cancer group showed a statistically signif i cantly higher treatment response rate and disease control rate. When second-line chemotherapy was compared with third-line or later chemotherapy, the median PFS of third-line or later chemotherapy was 2.25 months,which was statistically significantly longer than that with second-line therapy; however, the medial survival was not signif i cantly diffierent. A total of 11 patients were treated with pemetrexed as second-line therapy. Compared to 45 patients who received it as further-line therapy, the 11 patients who received second-line therapy were older and more frequently had squamous cell carcinoma (54.5% vs 40%). We believe that the longer PFS in the further-line pemetrexed group is explained by these diffierences in age and cancer type. In our study, only histology retained signif i cance in the multivariate analysis. Grade 3-4 hematologic toxicity occurred in 8.8% of cases. Although the present study included a large proportion of elderly and poor PS patients, the toxicity prof i les were mild and acceptable compared with the JMEI trial (a pemetrexed vs docetaxel trial)[6].
In a phase III clinical study examining the efficacy and safety of docetaxel and pemetrexed, prognostic factors included a PS of 0-1, stage III disease, and a relapse of >3 months after the last chemotherapy[6]. In the study conducted in Korea and reported by Lee et al[13], which assessed second-line chemotherapy for progressive NSCLC, pemetrexed monotherapy was administered for progressive NSCLC. The treatment response rate was 5.1%, and the disease control rate was 46.2%. The median survival was 7.8 months, and the median PFS was 3.1 months. Factors mediating the effiects on the treatment response rate were not determined, and independent factors mediating the effects on the disease control rate were non-smoker status and adenocarcinoma cancer type.
In another study reported by Lee et al[14], examining cases that used pemetrexed as second-line or later chemotherapy,the treatment response rate was 11%, and the disease control rate was 66%. The median survival was 13 months, and the median PFS was 2.3 months. In comparison with the squamous epithelial cancer group, the treatment response and disease control rates of the non-squamous epithelial cancer group were shown to be significantly higher, which was similar to our study.
In the study reported by Sun et al[15], the patients receiving pemetrexed were divided into two groups: the second-line group and the third-line or later group. The percentages in these two groups were 30% and 70%, respectively, which is comparable to our study, in which the ratio of third-line or later pemetrexed patients was 80.4%. The treatment response rate was 12%, and the disease control rate was 55%. The median survival in that study was 12.8 months, and the median PFS was 3.03 months. When the PFS for second-line chemotherapy was compared with that for with third-line chemotherapy,no signif i cant diffierence was detected. The only independent factor that showed a survival benef i t was PS.
Longer median PFS was found in the study reported by Lee, Sun, et al[14,15]and this was thought to be related to the fact that 79% and 59% of subjects were in PS levels 0 and 1, respectively, whereas in our study, 100% of cases were at PS levels 2 and higher. Thus, in the studies described above, as well as in our study, for NSCLC patients with poor PS, particularly for those with non-squamous cell cancer,pemetrexed is effiective as second-line or later chemotherapy.In cases when it is used as later than third-line chemotherapy,its efficacy was comparable to that for second-line therapy,and as in our study, for cases whose ECOG PS is >2, it was found to be efficacious.
