Microvessel density at different levels of normal or injured bile duct in dogs and its surgical implications
2011-06-11
Kunming,China
Introduction
Unprecedented attention to bile duct injury (BDI)has arisen since the introduction of laparoscopic cholecystectomy (LC).Over the past two decades,excellent outcomes after surgical repair have been reported by experienced teams.[1-3]Nevertheless,even in series from tertiary centers,failed repairs are still noted.[3-5]Proximal bile duct stricture is the main cause of a failed surgical repair of LC-related BDI.The correlation between ductal ischemia and recurrent stricture has been recognized.[6-8]Hepatic artery injury is believed to be an important contributor to a disappointing outcome.[8-10]However,good long-term outcomes are still obtained in cases of BDI concomitant with hepatic artery injury.[11]Arterial blood to the bile duct is directly supplied by the peribiliary plexus (PBP),and it is PBP damage,rather than hepatic artery injury,that leads to ductal ischemia.[11,12]PBP damage is more difficult to identify than hepatic artery injury in repair surgery; thus,ductal ischemia from underlying PBP damage can be neglected and followed by a post-repair bile duct stricture.It is not clear whether ischemia related to unidentified PBP damage results in failed repairs even when performed by experienced surgeons.If proximal ductal ischemia is a universal occurrence in BDI cases,it is unknown whether the severity of ischemia varies at different levels of the proximal bile duct.The answers to these questions are critical to decision-making in BDI repair.
The present study measured microvessel density(MVD) at different levels of normal and injured proximal bile ducts to investigate whether ischemia is universal and whether varied severity of ischemia at different levels of the proximal duct develops after BDI.
Methods
Animal model and grouping
The study was approved by the Institutional Review Board on Animal Experimentation of Kunming General Hospital of the Chengdu Military Area of the PLA.Thirty beagle dogs of either sex,weighing 15-23 kg,were randomly divided into control,BDI,and BDI-repaired groups.In 6 animals of the BDI group,a 1-cm segment of the common bile duct just above the upper border of the duodenum was carefully skeletonized.The duct was completely transected at the upper limit of the skeletonized segment to establish a modified model of the classic pattern of LC-related BDI (Fig.1).[13]A 3.5 Fr catheter with lateral bores,used as a draining tube,was inserted into the proximal bile duct via the transected end (Fig.2).The catheter was carefully secured to the bile duct with 3/0 silk,and the distal end was introduced into the duodenum andfixed.
Different from the human extrahepatic biliary system,the common bile duct in the beagle dog comes most commonly from the trifurcation of the three extrahepatically visible lobar ducts.The common bile duct varies in length.To guarantee comparability among samples,as well as facility of semi-circumferential ductal anastomosis,a ductal segment,from the transected level at the common duct to the level of the largest lobar duct close to the hilus,was trisected.The dividing lines were selected as the low (L),middle (M),and high (H) levels,depending on proximity to the transected level (Fig.1).Each animal was killed on postoperative day 15 and anterior semi-circumferencial ductal tissues at all three levels were sampled by microdissection.
Eighteen animals were included in the BDI-repaired group.Levels L,M,and H were selected as described in the BDI group.In each animal,after the BDI model was made,the anterior semi-circumference of the bile duct was incised at one of the three levels,then the incision was sutured with 7/0 prolene line over the intraductal catheter.The dogs were killed at postoperative day 15,and ductal tissue from one of the three levels was harvested as described in the BDI group.
In the control group,laparotomy was performed in six dogs only to expose the hepaticoduodeneal ligament.The virtual transected level was selected as the line 1 cm above the upper border of the duodenum,and levels L,M,and H were selected as above.Each animal was sacrificed on postoperative day 15 and anterior semicircumferencial ductal tissues from the three selected levels were harvested.
Each tissue specimen was divided into 3 fractions,in a volume of 2×2×1 mm,and 162 samples in total were collected.Dissection was carried out under 2.5×operative loupe magnification.
The samples werefixed in 10% formalin,embedded in paraffin and cut into 4 μm sections in preparion for measuring MVD by CD34 immunohistochemistry.The CD34 kit was from Maixin Biotechnology Development Co.,Fuzhou,China.The primary antibody was rice antihuman CD34 monoclonal antibody and the secondary antibody was HRP-conjugated goat anti-rice IgG.Yellow granules within the cytoplasm of the endoepithelial cells in the bile duct were defined as positive signals of CD34.MVDs were examined in 10 randomly and blindly chosenfields per section (Fig.3) and were calculated with a microspectrophotometer (Leica,Germany).[14]
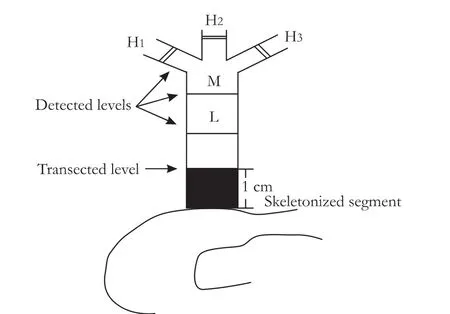
Fig.1.Schematic of the modified LC-related BDI model.Black section indicates skeletonized segment of the bile duct.
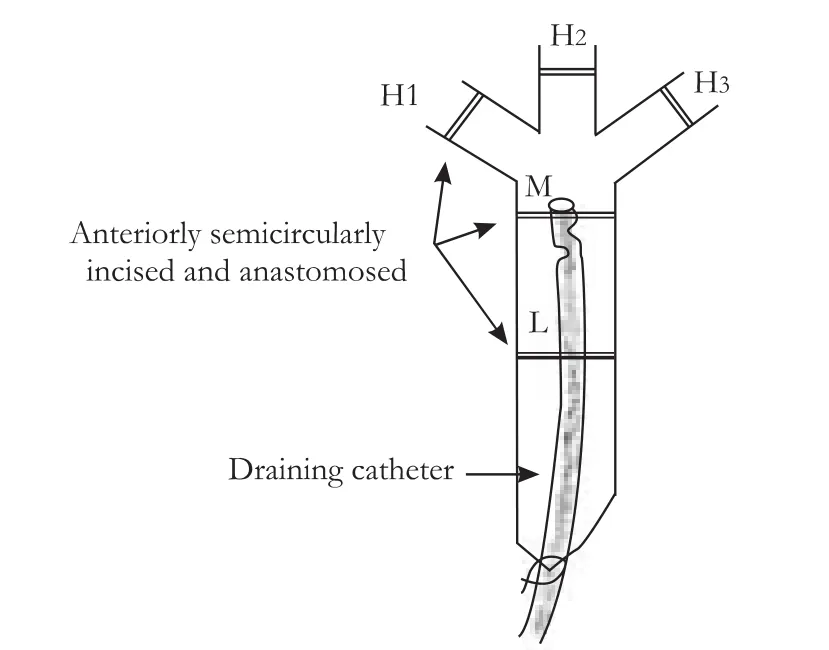
Fig.2.Injured proximal bile duct drained by a catheter with the distal end inserted into the duodenum.
Statistical analysis
Data were expressed as mean±SD and SPSS 13.0 software was used to analyze the data.The LSD t test and SNK inspection were used for analysis of the data.A P value less than 0.05 was considered to be statistically significant.
Results
MVDs at three levels of the bile duct
In the control group,MVD was unevenly distributed at the different levels of the normal extrahepatic bile duct.Despite the unremarkable difference between MVDs at levels H and M (P=0.053),as well as levels M and L(P=0.077),MVD at level H was significantly higher than that at level L (Table 1).The tendency was that the closer the level to the hilus,the higher the MVD at that level.In the BDI group,there was no significant difference between MVDs at levels M and L,but MVD at level H was clearly higher than that at both levels M and L (Table 2).In the BDI-repaired group,there were remarkable differences among MVDs at the three levels (Table 3).Our results showed that after BDI and BDI repair,the same tendency of uneven distribution of microvessels at the different levels of the proximal bile duct,as noted in the normal extrahepatic bile duct,were still found; the closer the level to the injury site,the lower was the MVD at that level.
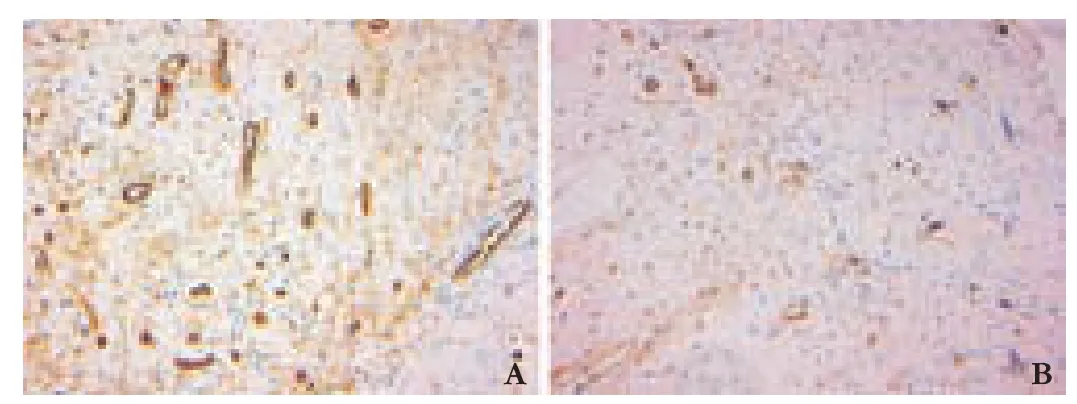
Fig.3.MVD at level L in the control group (A) and BDI group (B).(CD34 immunohistochemical staining,original magnification ×200).
Comparisons of MVDs at corresponding levels
After BDI or BDI repair,except for level H of the BDI group,MVDs at the corresponding level declined significantly compared with the control group (Table 4).Our findings suggested that universal ischemic damage of the bile duct had developed at the proximal bile duct,and the most serious ischemia occurred at the level close to the injury site.A relatively mild MVD decrease occurred distal to the injury site (level H).In addition,MVD at the each level in the BDI group was significantly lower than MVD at the corresponding level in the BDI-repaired group,suggesting that BDI repair worsened ductal ischemia.
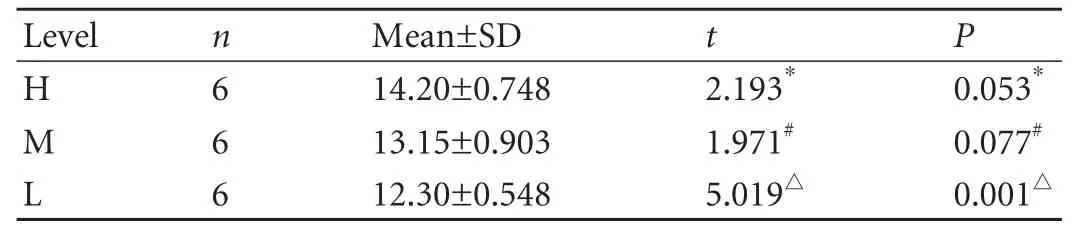
Table 1.MVDs at three levels of the bile duct in the control group
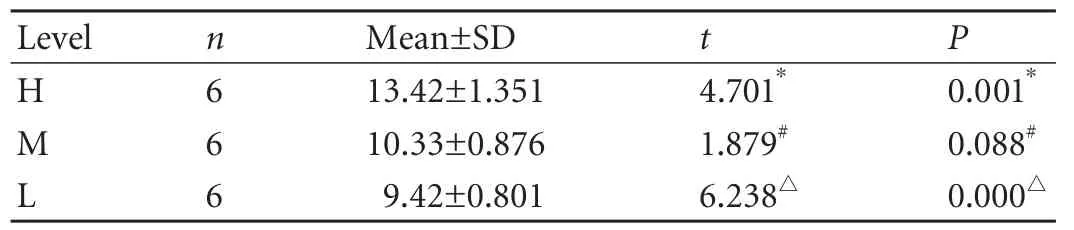
Table 2.MVDs at three levels of the bile duct in the BDI group
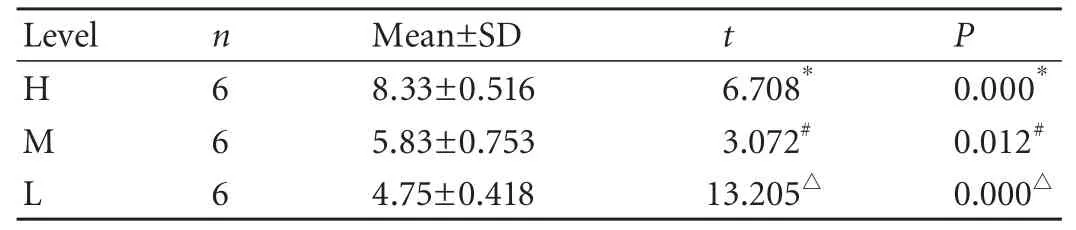
Table 3.MVDs at three levels of the bile duct in the BDI-repaired group

Table 4.Comparisons of MVDs at corrresponding levels
Discussion
Hepatic artery injuries were believed to be an important contributor to failed surgical repair of LC-BDI.[8-10]Concomitant vascular injuries were included in a recently proposed BDI classification system.[15,16]The exact incidence rate of hepatic artery injury in cases of LC-BDI is difficult to know.In a large series,the rate of hepatic artery injury was reported to be as high as 36%.[11]Based on an autopsy study in which the extrahepatic bile duct was intact,Northover[17]confirmed that 60% of the blood supply to the supraduodeneal bile duct comes from the ascending branches of the gastroduodenal artery,while 38% comes from the descending branches of the proper hepatic artery.Skeletonization and resection of a portion of the extrahepatic bile duct misidentified as the cystic duct is the classic pattern of LC-BDI.[13]In the process of skeletonizing and transecting the extrahepatic bile duct,mechanical and/or thermal injury to the PBP is inevitable.Thus,blood supply to the proximal bile duct comes only from the descending branches of the proper hepatic artery,as the ascending arterial supply to the duct proximal to the injury site would be greatly decreased.In fact,unilateral disruption of the right hepatic artery can be rapidly compensated for by the left hepatic artery via the hilar plate arterial plexus.[11]However,when the PBP is directly injured,ductal ischemia cannot be prevented.Certainly,PBP injury concomitant with complete disruption of the proper hepatic artery would worsen the ductal ischemia.In our BDI model,the hepatic artery was not injured,however,significant ischemia at the level close to the injury site was confirmed.Skeletonization and transection of the bile duct,which impaired the ascending blood supply from the PBP,was associated with ischemia at the level close to the injury site.
Terblanche and co-workers[7]found that high hepaticojejunostomy for bile duct strictures was better than low hepaticojejunostomy in terms of long-term outcome.Many retrospective reviews confirmed that failed primary surgical repair,in which the hepatic artery was injured or not,can be reversed successfully by higher biliary reconstruction.[2,10,11,18,19]Based on our findings,it is reasonable to believe that low biliary reconstruction at the ischemic low level of the proximal bile duct would lead to ischemic recurrent stricture,while high hepaticoenterostomy at a well-vascularized high level would give a satisfactory long-term outcome.
Two decades ago,Terblanche et al[7]concluded that the better arterial supply for the high proximal bile duct might be a major contributor to a better long-term outcome after high hepaticojejunostomy than after low biloenterostomy.Nevertheless,the authors did not confirm ischemia histologically at the low level of the proximal bile duct.With a modified LC-BDI model,we confirmed histologically that universal ischemia developed at the low level close to the injury site.The clinical implication of our study is that hepaticoenterostomy at or close to the injury site or end-to-end bile duct anastomosis might result in ischemic recurrent stricture of the proximal duct.In cases of BDI repair,no matter where the injury site is located,a high hepaticoenterostomy,such as the Hepp-Couinaud technique,high hepaticojejunostomy subsequent to hilar plate detachment or partial hepatectomy,[18-20]established at the well-vascularized high level of the proximal bile duct,are recommended.
Also,our results showed that MVD at the injured proximal duct further decreased after BDI repair,which indicated that great care should be taken to protect the PBP during repair of BDI.
Funding:The study was supported by a grant from the Eleventh Five-year Program for Medical Research of the PLA (06MB199).
Ethical approval:This study was approved by the institutional review board of the Kunming General Hospital of the Chengdu Military Area.
Contributors:LD proposed and designed the study.GL analyzed the data and wrote the first draft.All authors contributed to the interpretation of the study andfinal draft.LD is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Lillemoe KD,Melton GB,Cameron JL,Pitt HA,Campbell KA,Talamini MA,et al.Postoperative bile duct strictures:management and outcome in the 1990s.Ann Surg 2000;232:430-441.
2 Mercado MA,Chan C,Orozco H,Villalta JM,Barajas-Olivas A,Erana J,et al.Long-term evaluation of biliary reconstruction after partial resection of segments IV and V in iatrogenic injuries.J Gastrointest Surg 2006;10:77-82.
3 Schmidt SC,Langrehr JM,Hintze RE,Neuhaus P.Long-term results and risk factors in fluencing outcome of major bile duct injuries following cholecystectomy.Br J Surg 2005;92:76-82.
4 Walsh RM,Vogt DP,Ponsky JL,Brown N,Mascha E,Henderson JM.Management of failed biliary repairs for major bile duct injuries after laparoscopic cholecystectomy.J Am Coll Surg 2004;199:192-197.
5 de Reuver PR,Grossmann I,Busch OR,Obertop H,van Gulik TM,Gouma DJ.Referral pattern and timing of repair are risk factors for complications after reconstructive surgery for bile duct injury.Ann Surg 2007;245:763-770.
6 Stapleton GN,Hickman R,Terblanche J.Blood supply of the right and left hepatic ducts.Br J Surg 1998;85:202-207.
7 Terblanche J,Worthley CS,Spence RA,Krige JE.High or low hepaticojejunostomy for bile duct strictures? Surgery 1990;108:828-834.
8 Schmidt SC,Settmacher U,Langrehr JM,Neuhaus P.Management and outcome of patients with combined bile duct and hepatic arterial injuries after laparoscopic cholecystectomy.Surgery 2004;135:613-618.
9 Connor S,Garden OJ.Bile duct injury in the era of laparoscopic cholecystectomy.Br J Surg 2006;93:158-168.
10 Koffron A,Ferrario M,Parsons W,Nemcek A,Saker M,Abecassis M.Failed primary management of iatrogenic biliary injury:incidence and significance of concomitant hepatic arterial disruption.Surgery 2001;130:722-731.
11 Alves A,Farges O,Nicolet J,Watrin T,Sauvanet A,Belghiti J.Incidence and consequence of an hepatic artery injury in patients with postcholecystectomy bile duct strictures.Ann Surg 2003;238:93-96.
12 Deltenre P,Valla DC.Ischemic cholangiopathy.Semin Liver Dis 2008;28:235-246.
13 Davidoff AM,Pappas TN,Murray EA,Hilleren DJ,Johnson RD,Baker ME,et al.Mechanisms of major biliary injury during laparoscopic cholecystectomy.Ann Surg 1992;215:196-202.
14 Weidner N.Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors.Breast Cancer Res Treat 1995;36:169-180.
15 Lau WY,Lai EC.Classification of iatrogenic bile duct injury.Hepatobiliary Pancreat Dis Int 2007;6:459-463.
16 Bektas H,Schrem H,Winny M,Klempnauer J.Surgical treatment and outcome of iatrogenic bile duct lesions after cholecystectomy and the impact of different clinical classification systems.Br J Surg 2007;94:1119-1127.
17 Northover JM,Terblanche J.A new look at the arterial supply of the bile duct in man and its surgical implications.Br J Surg 1979;66:379-384.
18 Mercado MA,Chan C,Salgado-Nesme N,López-Rosales F.Intrahepatic repair of bile duct injuries.A comparative study.J Gastrointest Surg 2008;12:364-368.
19 Laurent A,Sauvanet A,Farges O,Watrin T,Rivkine E,Belghiti J.Major hepatectomy for the treatment of complex bile duct injury.Ann Surg 2008;248:77-83.
20 Thomson BN,Parks RW,Madhavan KK,Garden OJ.Liver resection and transplantation in the management of iatrogenic biliary injury.World J Surg 2007;31:2363-2369.
