Hemodynamics and oxygen transport dynamics during hepatic resection at different central venous pressures in a pig model
2011-06-11
Nanning,China
Introduction
Excessive hemorrhage and the need for blood transfusion are associated with increased postoperative morbidity and mortality and a worse long-term outcome.[1,2]In addition to use of the Pringle maneuver,an important advance that has contributed to reduced blood loss during hepatic resection is the use of low central venous pressure (CVP).Lowering CVP augments venous drainage from the liver,encourages blood flow away from the surgicalfield and minimizes blood loss.[3-10]A CVP <5 cmH2O is generally targeted by a combination of approaches including fluid volume restriction,pharmacologic manipulation (vasodilators,diuretics,and anesthetics),and body position (reverse Trendelenburg position).Although a low CVP is associated with reduced blood loss,it also carries an increased risk for complications such as air embolism,systemic tissue hypoperfusion resulting in stroke,myocardial infarction,and renal failure.Optimizing end-organ perfusion and minimizing blood loss during hepatectomy are the primary goals.However,the most effective level of CVP has not yet been evaluated.This study aimed to evaluate the impact of variations of low CVP on hemodynamic parameters and oxygen transport dynamics,as well as the rate of blood loss during partial hepatectomy in an animal model.
Methods
Instruments and agents
The following instruments and agents were used in this study:SC-M3 anesthesia respirator (Shanghai Medical Apparatus and Instrument Factory); Braun 50 microinfusion pump (Braun Co.,Germany); Hewlett-Packard patient monitor,model 54S (Hewlett-Packard Co.,USA); I-STAT mobile blood gas analyzer (I-STAT Co.,USA); internal jugular vein puncture package(Arrow Co.,USA); 7Fr Swan-Ganz catheter (Arrow Co.,USA); tracheal catheter (Cemma Enterprise Co.,Taiwan);EG7+andCG4+blood gas analysis agents (I-STAT Co.,USA).
Animals
Fifty miniature male or female Bama pigs,weighing 15-20 kg (17±2.1 kg) and 85-100 cm long (92±29 cm)were used.They were fasted for 12 hours and deprived of fluids for 6 hours before the operation.
Establishment of animal model of low CVP
Method of anesthesia
Ketamine (2 mg/kg) and diazepam (0.2 mg/kg) were used intramuscularly for basal anesthesia.A drip was set in the pig's ear vein and the following solution was infused with 500 mL 10% glucose,15 mL 10% KCl,10 mL 25%MgSO4,2 g inosine ,and 12 IU insulin.During induction of general anesthesia,ketamine (1 mg/kg),diazepam (0.2 mg/kg) and succinylcholine (2 mg/kg) were given.For the maintenance of anesthesia,250 mL Ringer's solution with 250 mg ketamine,25 mg diazepam and 250 mg succinylcholine was given intravenously.After endotracheal intubation,mechanical ventilation (tidal volume 10 mL/kg,respiration rate 14-16/min) was applied.The patient monitor was used to display the electrocardiogram and temperature.
Operative procedures (Fig.)
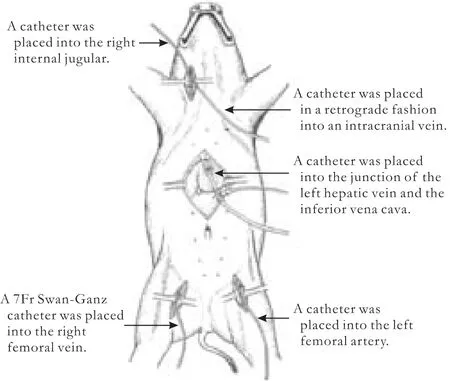
Fig.Establishment of pig model.
After sterilization,the left femoral artery was dissected,catheterized,and connected to a pressure transducer to monitor the mean arterial pressure(MAP).The right internal jugular vein was dissected,catheterized,and connected to the patient monitor to measure the CVP.A venous catheter was placed retrogradely into an intracranial vein to collect blood.The right femoral vein was isolated and a 7Fr Swan-Ganz catheter was inserted and connected to the patient monitor to record the pulmonary arterial pressure,pulmonary wedge pressure,cardiac output and heart rate.It was also used for collecting mixed venous blood.After opening the abdominal cavity,a left hepatic lobectomy was performed using a crush-clamp technique.The left portal vein was isolated to get venous samples.The left hepatic vein was isolated and the outer sheathing canal of a Swan-Ganz catheter was placed,with the debouch of the canal located at the junction of the left hepatic vein and the inferior vena cava.The catheter was then connected to a patient monitor to record the different hepatic venous pressures and blood loss per unit time at different levels of CVP.The temperature of the animal was controlled at 37 ℃.The arterial blood gas was assessed withEG7+agent.PaCO2was controlled at 35-45 mmHg by the anesthesia respirator.CVP was controlled in 8 groups (CVP 0-<1,1-<2,2-<3,3-<4,4-<5,5-<6,6-<7,and 7-<8 cmH2O groups) with different doses of nitroglycerin (0.4-0.8 μg/kg per minute ) regulated by a micro-mount infusion pump.
Data collection
The CVP was stabilized at different levels for 10 minutes.The hepatic venous pressure was monitored through the left hepatic vein catheter,which was then opened for 30 seconds to collect a blood sample and to measure the rate of blood loss.
Blood samples of 5 mL each were collected through the femoral artery (arterial blood),intracranial vein(intracranial venous blood) and Swan-Ganz catheter(mixed venous blood).Blood gases were analyzed by I-STAT.
Cardiac output (CO) was measured by the temperature dilution method through the Swan-Ganz catheter.Heart rate (HR) and MAP were recorded.Cardiac index (CI),arterial pO2(CaO2),oxygen delivery(DO2) and venous pO2(CvO2),oxygen consumption(VO2) and oxygen extraction ratio (ERO2) of mixed venous and intracranial venous samples were measured by the patient monitor.
The Institutional Animal Care and Use Committee approved this study protocol.
Statistical analysis
The data were expressed as mean±SD.SPSS software package version 13.0 was used for statistical analysis.Analysis of variance was used.The hepatic venous pressure and the blood loss through the hepatic vein were analyzed along with the CVP by linear correlation analysis,and the correlation coefficient r was calculated.P<0.05 was considered statistically significant.
Results
Of the 50 healthy Bama miniature pigs,two were excluded because of a failure of intubation and a failure of Swanz-Ganz catheter insertion,and at last 48 pigs were used for this study.
Hemodynamic changes
Differences in MAP,CO and CI between the CVP<2 cmH2O group and the CVP ≥2 cmH2O group were statistically significant (Table 1).A significant drop in MAP,CO and CI was seen.Blood loss and hepatic venous pressure during hepatic resection were almost linearly related to the CVP level,whereas blood loss in the CVP ≥5 cmH2O group was more significant than in the CVP <5 cmH2O group (Table 2).
Oxygen transport dynamics
The DO2,VO2and ERO2remained relatively constant for CVP levels from 2 to <8 cmH2O.The difference in DO2between the CVP <2 cmH2O group and the CVP ≥2 cmH2O group was significant,as it was in VO2and ERO2between the CVP <1 cmH2O group and the CVP ≥1 cmH2O group (Table 3).

Table 1.Hemodynamic changes at different levels of CVP

Table 2.Hepatic vein pressure and blood loss at different levels of CVP
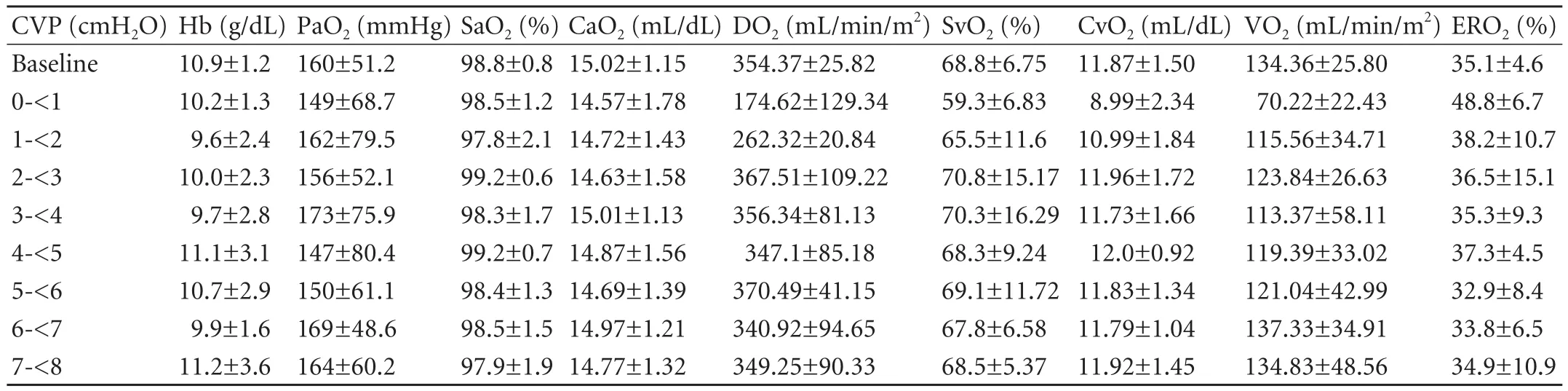
Table 3.Oxygen transport dynamics at different levels of CVP

Table 4.Oxygen metabolic index in brain of different levels of CVP
In fluence of brain oxygen metabolism index due to different levels of CVP
Venous oxygen saturation (SvO2),CvO2and VO2were significantly lower in the CVP <1 cmH2O group than in the CVP ≥1 cmH2O group.ERO2was significantly higher in the CVP <1 cmH2O group than in the CVP ≥1 cmH2O group (Table 4).
Discussion
Any strategy to reduce blood loss during hepatic resection and to decrease blood transfusion would be of benefit to patients.The volume of blood loss during hepatic resection correlates with the CVP.The maintenance of low CVP during the operation precludes vena caval distention and facilitates mobilization of the liver and dissection of the retrohepatic and major hepatic veins.More importantly,the low CVP approach minimizes hepatic venous bleeding during parenchymal transection and facilitates control of any inadvertent venous injury,particularly to the intrahepatic part of the middle hepatic vein.Management should aim to keep the CVP low without subjecting the patient to the risk of air embolism and systemic tissue hypoperfusion.
In this animal model,we studied the hemodynamic effects and oxygen transport properties at different levels of the CVP.Our findings are in accord with the studies showing blood loss during hepatic resection,which is almost linearly related to the CVP level.[6-9]
It is reasonable to assume that below a critical level,VO2is inversely related to the risk of cellular dysfunction and necrosis as well as to the severity of hypoperfusion.Among the various hemodynamic variables that may be evaluated,a VO2below the required level is most strongly related to death.Once a substantial amount of cell necrosis has occurred,functional recovery of the organ becomes impossible,even when adequate VO2is restored.For all systems,VO2is different between the input flow and the output flow.For the circulation of the whole body,the input flow is the arterial DO2and the output flow is the venous oxygen delivery.If one considers the ERO2to be the ratio between VO2and DO2,then VO2can be represented by the product DO2×ERO2.The simple equation VO2=DO2×ERO2is conventionally used to represent the macrocirculatory balance.Direct assessment of the VO2/DO2relationship is a simple way to evaluate the existence of a gap between the calculated VO2at a specific point in time and the adequacy of VO2.[11,12]Under normal conditions,a change in DO2does not result in a similar change in VO2.However,when DO2decreases to a critical value,a change in VO2depends on a change in DO2.When a further increase of ERO2cannot satisfy tissue oxygenation,anaerobic metabolism sets in.In this experiment,DO2decreased when CVP <2 cmH2O,meanwhile the mixed venous VO2in the CVP <1 cmH2O group was less than in the other groups,and ERO2in the CVP <1 cmH2O group was more than in the other groups.
A negative CVP can rapidly allow large volumes of air through small or unrecognized lacerations of the hepatic veins.This is unlikely with a positive CVP.
In conclusion,in this study the rate of blood loss and the hepatic venous pressure during hepatic resection were almost linearly related to the CVP (r=0.933,P<0.001); hence the lower the CVP,the less the blood loss.Moreover there were significant differences in CO,CI and DO2between the CVP <2 cmH2O group and the CVP ≥2 cmH2O group.When the CVP was<2 cmH2O,there was hemodynamic instability with a significantly lower CO value and a significantly lower ventricular filling pressure.As a result,a CVP at 2 to 3 cmH2O was found to be optimal for hepatic resection as it decreased blood loss while maintaining the stability of hemodynamics and oxygen metabolism.
Funding:This study was supported by a grant from the Guangxi Natural Science Foundation (GKZ0447066).
Ethical approval:Not needed.
Contributors:GY and LWY proposed the study.GY wrote the first draft.All authors contributed to the design and interpretation of the study and to further drafts.GY is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 de Boer MT,Molenaar IQ,Porte RJ.Impact of blood loss on outcome after liver resection.Dig Surg 2007;24:259-264.
2 Kooby DA,Stockman J,Ben-Porat L,Gonen M,Jarnagin WR,Dematteo RP,et al.In fluence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases.Ann Surg 2003;237:860-870.
3 Muller MK,Petrowsky H,Clavien PA.Techniques of Vascular Control and Protective Strategies for Parenchymal Transection.In Lau WY,ed.Hepatocellular Carcinoma:World Scientific Publishing Co.Pte.Ltd.;2008:507-528.
4 Lau WY.A review on the operative techniques in liver resection.Chin Med J (Engl) 1997;110:567-570.
5 Chui AK,Moultan CE,Lau WY.Trendelenburg patient positioning:a reevaluation.J Am Coll Surg 2000;190:760-761.
6 Lai PB,Chui PT,Leow CK,Lau WY.Correlation between blood loss and inferior vena caval pressure during liver resection.Br J Surg 1998;85:1158.
7 Jones RM,Moulton CE,Hardy KJ.Central venous pressure and its effect on blood loss during liver resection.Br J Surg 1998;85:1058-1060.
8 Melendez JA,Arslan V,Fischer ME,Wuest D,JarnaginWR,Fong Y,et al.Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia:blood loss,blood transfusion,and the risk of postoperative renal dysfunction.J Am Coll Surg 1998;187:620-625.
9 Wang WD,Liang LJ,Huang XQ,Yin XY.Low central venous pressure reduces blood loss in hepatectomy.World J Gastroenterol 2006;12:935-939.
10 Gurusamy KS,Li J,Sharma D,Davidson BR.Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection.Cochrane Database Syst Rev 2009;(4):CD007338.
11 Caille V,Squara P.Oxygen uptake-to-delivery relationship:a way to assess adequate flow.Crit Care 2006;10:S4.
12 Squara P.Matching total body oxygen consumption and delivery:a crucial objective? Intensive Care Med 2004;30:2170-2179.
