Expression profile of cholecystokinin type-A receptor in gallbladder cancer and gallstone disease
2011-06-11
Varanasi,India
Introduction
Gallbladder cancer (GBC) is a highly aggressive and incurable disease representing the most common malignancy of the biliary tract with a threefold higher incidence in females.[1,2]GBC shows striking geographical predilections in its incidence,with the highestfigures found in India and Chile and relatively low levels in many Western countries.[3]In North India,it is the third most common malignancy in females with an overall 5-year survival of less than 5%.[4]The dismal outcome for GBC is ascribed to late presentation in the absence of specific symptoms,limited knowledge of its etiology,rapid and silent progression,and ineffective therapy (surgical resection,chemotherapy and radiotherapy).To date,GBC continues to be a challenge for oncologists with regard to early-stage diagnosis and optimal management of advanced disease,and warrants continuous evaluation of all factors including molecular markers associated with the disease.These include ER/PR,HER-2,p53,CEA,CA19-9,cyclin D1,p16,and Rb.Another such approach might be hormonal manipulation which is currently limited to breast,prostate and thyroid cancer.In this broad approach,how the basic hormone cholecystokinin (CCK) affects the function of the gallbladder has not been studied.
Over the past few years,a radiolabeled somatostatin analog I has been widely used to detect human neuroendocrine tumors and their metastases,since somatostatin receptors are over-expressed in these tumors.Now this receptor is considered as a target to treat these tumors.It is known that other regulatory peptides and their receptors are also more highly expressed in neoplastic cells than in the normal tissue of origin or nearby normal tissue.[5]Today,this area of targeting human cancer by specific peptide receptors either for diagnosis or for therapeutic reasons (by exogenous administration of corresponding peptides/peptide analogues) is a subject of intense investigation and considered a promising strategy in oncology,compared with earlier,less-specific approaches.[6]
CCK and gastrin are members of a group of regulatory peptide hormones having a wide range of physiological actions in the gastrointestinal tract,mediated by their receptors distributed throughout the tract.In addition to promoting normal cell proliferation,they have also been shown to stimulate the growth of several neoplasms and their receptors have been found to be expressed in a variety of human neoplastic tissues.[7,8]For this reason,these growth factors and changes in their receptors have become important in oncology.Further,CCK is a potent modulator of gallbladder motility by activating CCK type-A receptor (CCKAR) distributed on gallbladder smooth muscle cells[9]and abnormal processing of the CCKAR gene is associated with gallbladder disease.[10]Lowered expression of CCKAR contributes to the hypomotility of the gallbladder in GSD patients,[11]but no work has been done regarding the expression of CCKAR in GBC.These observations have augmented our interest to investigate the CCKAR expression in GSD/GBC in this part of the world with a high incidence of GBC and GSD.
It was not envisaged that CCKAR would show any dramatic prevalence in patients with GBC and GSD.We therefore looked for any change in the expression profile of CCKAR which may indicate any carcinogenic signs or any prognostic significance.The aim of this pilot study was to establish a relationship between CCKAR and the presence of GBC.
Methods
Patients
This case-control study included patients undergoing surgery for GBC and GSD from January 2007 to January 2009 in the Department of Surgical Oncology,Sir Sunderlal Hospital,Banaras Hindu University,a tertiary care hospital in North India.The study was approved by the institutional Ethics Committee.
Since the proportion of operated GBC cases was higher in this study,we planned to take GBC cases as 1.25 times those of GSD.The sample sizes were calculated taking the prevalence of GBC=47 and GSD=25,level of significance=5% and power of test= 80%.Thus the minimum number of samples required for the study was 93 for GBC and 74 for GSD.A total of 94 GBC and 68 GSD samples were included because of the poor immunohistochemistry staining of some GSD samples.Informed consent,a detailed history and imaging of the abdomen of each patient were also noted on the proforma.
Inclusion criteria and exclusion criteria
Histologically proven GBC (cases) and GSD (controls)and those who consented to participate in the study were included in this study protocol.
Immunohistology
The tissue samples obtained from fresh surgically resected gallbladder specimens werefixed in 10%buffered formalin and embedded in paraffin.Three to four micrometer sections were cut and stained with hematoxylin and eosin (HE).The HE sections were evaluated under a light microscope for histopathological diagnosis and grading.
Separate 3-4 μm sections were cut and placed on polylysine-coated slides and used for immunohistochemical staining according to the manufacturer's instructions.Primary antibody and a secondary kit used for detection of CCKAR (CCK-AR (H-60) antibody; sc-33220) were from Santa Cruz,and the Super Sensitive Link-Lable IHC Detection System (QD000-5L) was from BiogGenex,San Ramon,CA,USA.
Brie fly,all sections were dewaxed and rehydrated in xylene and graded alcohol,and placed under slowrunning tap water for 15 minutes followed by citrate buffer (pH 6.0) retrieval by the microwave method.The sections were allowed to cool at room temperature and washed 3 times with Tris buffer (TBS,pH 7.6).Then they were incubated with peroxidase block for 20 minutes to check internal peroxidase activity.After washing with TBS,these sections were incubated with power block for 15 minutes to block nonspecific staining.Excess power block was removed and then the sections were immediately incubated with primary antibody at 1∶200 dilution in TBS overnight at 4 ℃.After washing with TBS,the sections were incubated with multilink for 30 minutes and washed with TBS followed by secondary antibody incubation again for 30 minutes.After washing,color was developed using diaminobenzidine(DAB) as the chromogen.Finally,slides were washed and counterstained with Mayer's hematoxylin and mounted with DPX (a mounting medium containing mixture of Distyrene,a plasticizer and xylene).All the incubation steps were carried out in a moist chamber to avoid drying of sections.
For positive control,a section known to stain positively on the gallbladder muscle layer component was included in each batch of staining,and for negative control primary antibody was replaced with TBS.
Evaluation of staining pattern
The results were evaluated quantitatively as well as qualitatively according to the intensity of staining pattern by the scoring system used for breast cancer,as there is no standard scoring system for gallbladder cancer.Intensity of staining was graded as negative (0),weak (1),moderate (2) or strong (3).The percentage of cells showing staining was graded as:none (0),1 (<1%),2 (1%-10%),3 (11%-33%),4 (34%-66%) or 5 (>66%).Total staining score was calculated by adding the intensity score and the percentage score as negative (0),weak/+ (2),moderate/2+ (3-5),or strong/3+ (6-8).[12]
Immunoblotting
For the immunoblotting study,we randomly selected 16 samples each of GBC and GSD.Isolation of whole-cell protein was done from formalin-fixed paraffin-embedded blocks using the Qproteome FFPE tissue kit (Qiagen).Protein concentration was determined by the Bradford assay.[13]Thirty micrograms of total protein from each GBC and GSD sample was boiled for 10 minutes in denaturing sample buffer (10% glycerol,1% SDS,1%β-mercaptoethanol,10 mmol/L Tris-HCl (pH 6.8),and 0.01% bromophenol blue),resolved on 12% acrylamide gels,and transferred to Immuno-BlotTMpolyvinylidene di fluoridefilter membranes (Millipore).Non-specific sites were blocked with 5% skimmed milk for 2 hours at room temperature and then incubated overnight at 4 ℃with primary antibody (1∶5000 dilution) for CCKAR(Santa Cruz).Subsequently,the blots were washed three times in 0.1% Tween-20 in TBS and then incubated with a 1∶10 000 dilution of secondary antibody (HRP conjugate)for 1 hour at 25 ℃.After extensive washing with 0.1%Tween-20 in TBS,substrate solution was added to the membrane,incubated for 5-15 seconds and exposed at room temperature.The membranes were developed with an enhanced chemiluminescence kit,following the manufacturer's instructions (GE Healthcare,USA).For normalization,the membranes were stripped using buffer containing 62.5 mmol/L Tris,pH 6.8,2% SDS,and 100 mmol/L β-mercaptoethanol and then re-probed with anti-β-actin antibody (Santa Cruz) at 1∶10 000 dilution.Quantification of band intensity was performed by densitometry using Quantity One software (v.4.5.1) and a Gel Doc imaging system (Bio-Rad).Expression of CCKAR was semiquantified as absent,faint-medium or strong.
Statistical analysis
Data were analysed using the Statistical Package for Social Sciences version 15 (SPSS,Chicago,IL,USA) and MSTAT.The association between receptor expression and clinical history of the patients was evaluated using Fisher's exact probability test and the Mann-Whitney U test.A difference was considered significant at the 5% level.
Results
The mean age of the 94 GBC cases was 54.22±11.23 years(range 30-76) and that of the 68 GSD controls was 48.03±11.04 years (range 27-65).Although the GBC cases had a higher mean age than the GSD controls,this difference was not statistically significant.The male to female ratio was 1∶2.6 for GBC and 1∶3.5 for GSD patients.
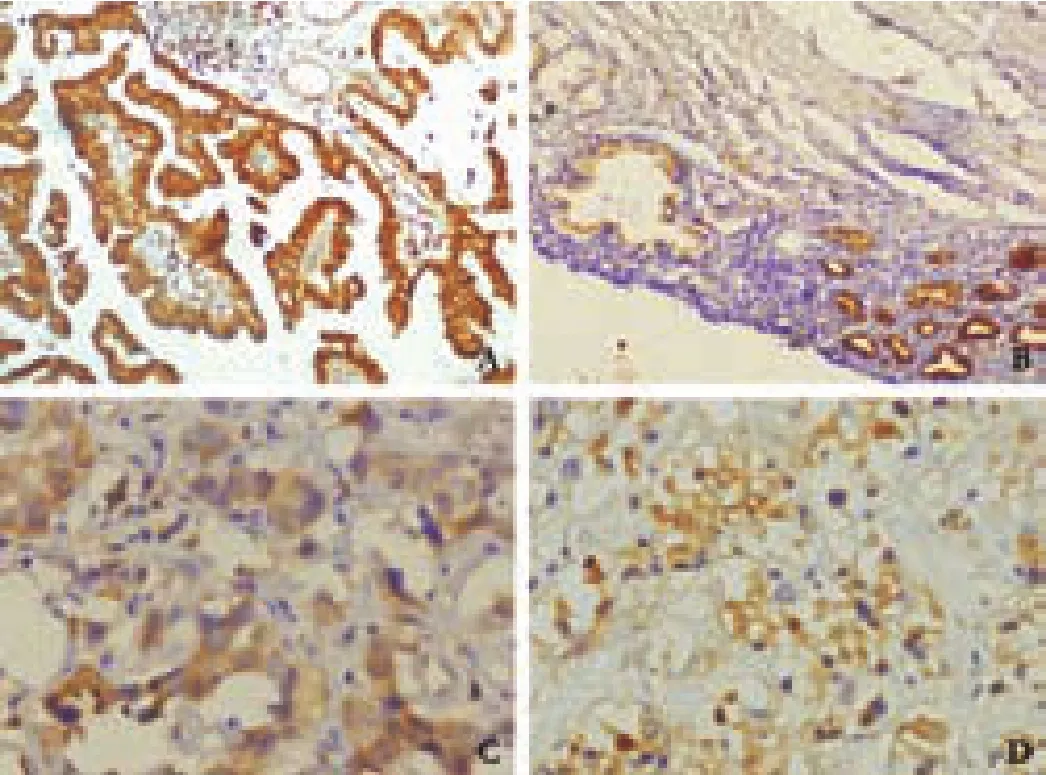
Fig.1.CCKAR positivity in GBC.A:strong positivity in welldifferentiated GBC; B:focal strong positivity in normal and metaplastic glands; C:moderate positivity in moderatelydifferentiated GBC; D:moderate positivity in poorly-differentiated GBC.CCKAR:cholecystokinin type-A receptor; GBC:gallbladder cancer.

Fig.2.CCKAR positivity in gallbladder muscle (A,positive control),GSD excluding primary antibody (B,negative control),GSD (C),and GSD with polyp (D).CCKAR:cholecystokinin type-A receptor; GSD:gallstone disease.
Positive CCKAR immuno-reactivity was found mainly in the cytosol of the epithelial mucosa and muscle layer of GBC and GSD samples (Figs.1,2).In some samples of GBC and GSD,a focal intense granular staining pattern was also observed in normal,neoplastic and metaplastic glands lined by epithelial mucosa.CCKAR expression was detected in 30 (44.1%) of the 68 GSD samples (controls) while it was found in 72 (76.6%)of 94 GBC samples.There was a significant difference between the two groups (Table 1).Most of the GSD controls showed moderate or weak positivity (43.3%),while strong positivity was observed only in 13.3% of the samples.In contrast,51 (51/72,70.8%) of GBC cases showed strong positivity and only few cases had moderate(22.2%) or weak (6.9%) positivity (Fig.3).Weak to moderate positivity on smooth muscle cells was observed in 36.76% of GSD cases and 21.27% of GBC cases.
Immunoblotting of CCKAR protein was performed to substantiate the immunohistology results.Immunoblotting of tissue samples,using a specific anti-CCKAR antibody,showed an equal 85 kDa protein band (Fig.4A).Similarly,immunoblotting also revealed frequent expression of CCKAR protein in GBC cases (14/16,87.5%) as compared to GSD cases (8/16,50%); there was a significant difference (P=0.02) between the two groups.In addition,the average CCKAR protein expression wassignificantly higher in GBC tissue (1.62±0.24) than in GSD tissue (0.49±0.08,P<0.001,Fig.4B).Strong expression of CCKAR protein was found more in GBC(11/16,68.75%) than in GSD cases (2/16,12.5%),while faint-moderate expression predominated in GSD (6/16,37.5%) over GBC cases (3/16,18.75%).
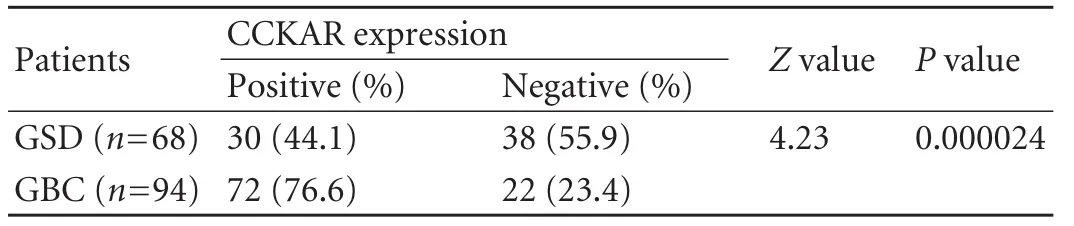
Table 1.CCKAR expression by immunohistochemistry in GSD(controls) and GBC (cases)
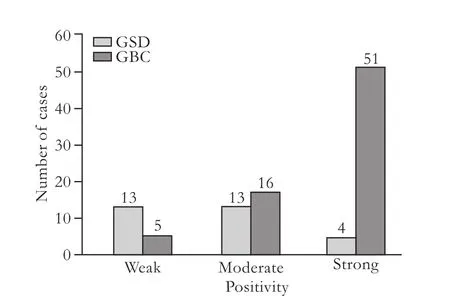
Fig.3.Number of cases showing weak,moderate and strong positivity in both GBC and GSD groups.GBC:gallbladder cancer;GSD:gallstone disease.
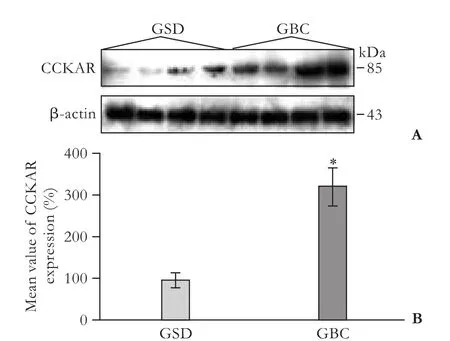
Fig.4.A:Representative immunoblots showing protein level of CCKAR in GSD and GBC samples.GBC samples showed significantly higher levels of CCKAR protein; B:average value of CCKAR protein expression (in percent) in 16 samples each of GSD and GBC studied by immunoblotting.The level of CCKAR expression was quantified as the ratio of the intensity of the CCKAR signal to that of β-actin.The average CCKAR expression in GBC samples was significantly higher than that in GSD samples(P<0.001).The results are presented as mean±SE (n=16).CCKAR:cholecystokinin type-A receptor; GBC:gallbladder cancer; GSD:gallstone disease; *:P<0.001.
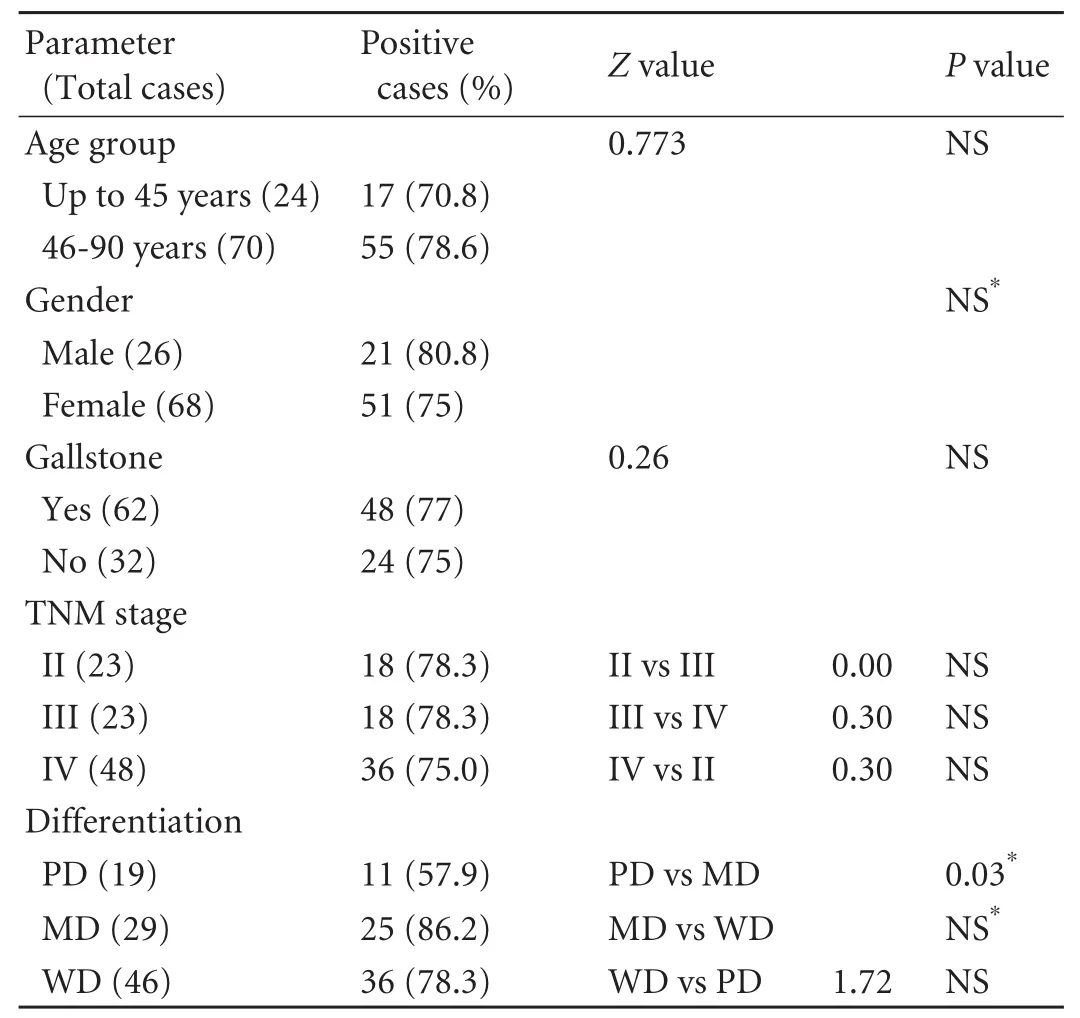
Table 2.Correlation of CCKAR expression with clinical history of GBC patients
The correlation between CCKAR expression and clinical parameters in GBC cases is depicted in Table 2.A higher CCKAR expression was found in those over 45 years,in males,and with the presence of gallstone,although the differences were not statistically significant.No correlation of CCKAR positivity with the clinical stage was found.In relation to differentiation of tumors,57.9% of poorly-differentiated (PD),86.2%of moderately-differentiated (MD) and 78.3% of welldifferentiated (WD) samples of GBC revealed CCKAR expression; however a statistically significant difference only occurred between the PD and MD samples of GBC(Table 2).Further,a higher prevalence/strong positivity of CCKAR expression was found to be associated mainly with MD and WD samples of GBC as compared to PD.
Discussion
GBC is usually fatal and thefifth most common malignancy of the gastrointestinal tract in the United States[14]having a tendency to propagate early.Like many other tumors,GBC is not associated with a defined tumor marker and specific symptoms for diagnosis.About 70%-80% of GBC cases are detected at an advanced stage.At present,there is no effective treatment other than surgery at early stages of the disease.
CCK is one of the longest-known hormones released from endocrine cells of the small intestine.Initially it was identified as an important factor for controlling gallbladder motility and pancreatic enzyme secretion.[15]Increasing evidence has demonstrated a trophic effect of CCK on the pancreas,gastrointestinal mucosa and epithelial cells of the gallbladder.[16,17]All these physiological actions of CCK are mediated by G-proteincoupled receptors,either the CCKAR or the CCCKBR,which have 48% structural homology,though CCKAR has higher affinity for sulphated CCK than gastrin/nonsulphated CCK.CCKARs are predominantly distributed in the gallbladder,pancreas and brain while CCK-B/gastrin receptors are present in the gut mucosa and brain.[18]CCK is also a well-known growth factor for pancreatic hyperplasia and induces carcinogen-mediated carcinogenesis of acinar tumors in the pancreas by activating CCKARs.[19]In addition,CCKARs are expressed in a variety of human tumors,primarily in significant numbers of gastroenteropancreatic tumors,meningiomas,and neuroblastomas.[20]Weinberg et al[21]found expression of CCKARs in pancreatic carcinoma,suggesting this as a marker of pancreatic carcinogenesis in humans.Further,caerulein,a CCK analogue,potentiates the growth of CCKAR-positive human pancreatic carcinoma,though it has no effect on those lacking CCK receptors.[22]These observations advocate the possibility of a trophic action of CCK through CCKARs in GBC which could be used for diagnostic as well as therapeutic purposes.Investigation of the expression of these regulatory peptides and their receptors in human tumors may help in opening a new front for cancer prognosis and prevention.However,there is no literature available on expression analysis of CCKAR in GBC.Hence,this study was conducted to analyze the expression profile of CCKARs in gallbladder disease and their prognostic significance.
Our immunohistochemical study demonstrated CCKAR positivity in 44.1% of GSD and 76.6% of GBC samples with a significant difference.The GSD showed mainly weak and moderate expression (43.3%),though a sample of GSD with polyp showed intense CCKAR positivity in nearly all gallbladder mucosa with some focal granular pattern of positivity (Fig.2D).On the contrary,70.8% of CCKAR-positive GBC cases revealed over-expression (Fig.1).Similarly,in the immunoblotting study we found that the level of CCKAR expression was significantly higher in GBC than in GSD.CCKAR positivity was found in normal,neoplastic and metaplastic glands of GBC.This over-expression may be due to up-regulation of CCKAR mRNA.Up-regulation of CCKAR mRNA has also been shown in animals fed a high cholesterol diet,which is associated with decreased contractility of the gallbladder.[23]Abnormal expression of CCK receptors in the gallbladder mucosal epithelium has also been shown in patients with chronic acalculous gallbladder dysfunction compared with normal healthy controls,suggesting that up-regulation of CCK receptor mRNA in the gallbladder may be due to impaired CCK signaling related to increased membrane cholesterol content and a related reaction of biological compensation to increase the receptor concentration.[24]
On the other hand,lack of CCKARs has been found to promote gallstone formation.[25]Further,abnormal or decreased expression of CCKAR in the smooth muscle layer is associated with gallbladder motility disorder.Miyasaka et al[26]also reported decreased expression of CCKAR in the gallbladder muscle component associated with gallstone as compared to those without gallstone.Similarly,we found decreased expression of CCKAR in the muscle layer of GBC and GSD.This discrepancy in our results may be due to that most of the studies are based on the reverse transcription polymerase chain reaction or receptor autoradiography involving only gallbladder muscle.Immunohistochemical study of CCKAR on the whole gallbladder tissue still needs to be explored.
Various studies have also shown CCKAR mRNA expression in gastric,esophageal,pancreatic and colon cancer,but the results are controversial.Expression of CCK and CCKAR mRNA occurs in 29% and 36%of gastric cancer,respectively,while 21% of gastric cancer shows co-expression of both CCK and CCKAR,suggesting autocrine regulation of tumor growth by CCK.[27]Clerc et al[28]found CCKAR gene expression in most esophageal (62%),gastric (62%) and colon(42%) cancers,indicating the in fluence of CCK of local or systemic origin on the growth of these tumors.Further,they also found that the development of esophageal cancer is associated with increased CCKAR gene expression both in tumor and surrounding cells.Increasing evidence shows the involvement of CCK and CCKARs in pancreatic carcinogenesis.[29]Another study[30]revealed immunohistochemical expression of CCKAR in human insulinomas,carcinoids,pituitary adenomas and meningiomas.
We found a gradual increase in CCKAR expression from GSD to GBC.Since long-standing gallstones have been attributed to the pathogenesis of GBC,[31,32]it seems that aberrant CCKAR expression is associated with disease progression from cholelithiasis to early carcinoma.As predicted by Lamote et al,[17]our results also suggest the involvement of a trophic action of CCK on the gallbladder mucosa through the CCKAR,and its over-expression in GBC may be a matter for further investigation.Further,to our knowledge,this is the first immunohistochemical evaluation of CCKAR expression in the gallbladder.
Analysis of the relationship between CCKAR expression and clinical parameters revealed no significant association between CCKAR expression and age,gender and gallstones.The prevalence of CCKAR expression did not differ much with the clinical stage of GBC.The CCKAR positivity increased with tumor differentiation from PD to MD and WD samples of GBC,while a statistically significant difference occurred only between PD and MD samples.Interestingly,overexpression of CCKAR was significantly associated mainly with WD and MD samples in comparison to PD samples (Fig.1).Our results are compatible with those of Okada et al,[27]who reported CCKAR gene expression in 50% of histological type while only one poorlydifferentiated adenocarcinoma showed expression,suggesting that dedifferentiation and loss of tissuespecific receptors are more often associated with the poorly-differentiated type of cancer.Similarly,higher expression of CCKBR occurs in differentiated gastric cancer than in the undifferentiated type.[33]
However,our study has some limitations as it used a single marker protein,and did not investigate the expression of CCKARs in normal gallbladder or epithelial dysplasia.Since CCKAR expression is also found in a myriad of gastrointestinal disorders,CCKAR involvement in pathogenesis is only a part of the overall puzzle.Further research is warranted with much larger samples for detection of CCKAR in normal gallbladder and in epithelial dysplasia to determine whether CCKAR expression progressively increases with the onset of changes in the gallbladder epithelium.Moreover,coexpression of CCK,in situ detection of CCKAR,the mechanism of its up-regulation,and secondary signalling pathways linked to it,should also be examined to explore the involvement of this receptor in the development and progression of GBC.
In conclusion,our findings showed a significant physiological variation in the expression of CCKAR in gallbladder disease,providing a new avenue of research.CCK is admittedly one of many regulatory peptides or hormones involved in this disease.Our observation indicates that CCKAR is significantly overexpressed in GBC and encourages the inclusion of more peptides and hormones in future studies.In addition,the over-expression of CCKARs in most cases of GBC may suggest the use of receptor antagonists for tumor localization,clinical assessment,and/or receptor-based delivery of therapeutic agents (cytotoxic toxin linked to a specific ligand of these receptors) to treat GBC.
Acknowledgment
We are grateful to Vishal Chandra (Division of Endocrinology,Central Drug Research Institute Lucknow) and Shashikant Patane(Department of Pathology,Institute of Medical Sciences,BHU,Varanasi) for their kind help and support.
Funding:This work was supported by a grant from the Indian Council of Medical Research (ICMR Project Ref No.3/2/2/187/2009/NCD-III).
Ethical approval:The study was approved by the Ethics Committee of the Institute of Medical Sciences,Banaras Hindu University,India.
Contributors:SHS proposed the study.RR wrote the first draft under the supervision of TM.STB analyzed the data.All authors contributed to the design and interpretation of the study and to further drafts.SHS is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Pesic M,Karanikolic A,Djordjevic N,Gmijović D,Bašić H.Clinical characteristics of primary carcinoma of the gallbladder.Facta Universitatis.Series:Medicine and Biology 2002;9:227-230.
2 Randi G,Franceschi S,La Vecchia C.Gallbladder cancer worldwide:geographical distribution and risk factors.Int J Cancer 2006;118:1591-1602.
3 Goldin RD,Roa JC.Gallbladder cancer:a morphological and molecular update.Histopathology 2009;55:218-229.
4 Shukla HS,Awasthi K,Naithani YP,Gupta SC.A clinicopathological study of carcinoma of the gall bladder.Indian J Cancer 1981;18:198-201.
5 Reubi JC.Peptide receptors as molecular targets for cancer diagnosis and therapy.Endocr Rev 2003;24:389-427.
6 Reubi JC,Maecke HR.Peptide-based probes for cancer imaging.J Nucl Med 2008;49:1735-1738.
7 Thomas RP,Hellmich MR,Townsend CM Jr,Evers BM.Role of gastrointestinal hormones in the proliferation of normal and neoplastic tissues.Endocr Rev 2003;24:571-599.
8 Baldwin GS,Shulkes A.CCK receptors and cancer.Curr Top Med Chem 2007;7:1232-1238.
9 Ivy AC,Oldberg A.A hormone mechanism for gallbladder contraction and evacuation.Am J Physiol 1928;86:599-613.
10 Miller LJ,Holicky EL,Ulrich CD,Wieben ED.Abnormal processing of the human cholecystokinin receptor gene in association with gallstones and obesity.Gastroenterology 1995;109:1375-1380.
11 Ding X,Lu CY,Mei Y,Liu CA,Shi YJ.Correlation between gene expression of CCK-A receptor and emptying dysfunction of the gallbladder in patients with gallstones and diabetes mellitus.Hepatobiliary Pancreat Dis Int 2005;4:295-298.
12 Allred DC,Harvey JM,Berardo M,Clark GM.Prognostic and predictive factors in breast cancer by immunohistochemical analysis.Mod Pathol 1998;11:155-168.
13 Bradford MM.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal Biochem 1976;72:248-254.
14 Pitt HA,Dooley WC,Yeo CJ,Cameron JL.Malignancies of the biliary tree.Curr Probl Surg 1995;32:1-90.
15 Rehfeld JF.Clinical endocrinology and metabolism.Cholecystokinin.Best Pract Res Clin Endocrinol Metab 2004;18:569-586.
16 Guo YS,Townsend CM Jr.Roles of gastrointestinal hormones in pancreatic cancer.J Hepatobiliary Pancreat Surg 2000;7:276-285.
17 Lamote J,Putz P,Willems G.Effect of cholecystokininoctapeptide,caerulein,and pentagastrin on epithelial cell proliferation in the murine gallbladder.Gastroenterology 1982;83:371-377.
18 Wank SA.Cholecystokinin receptors.Am J Physiol 1995;269:G628-646.
19 Baldwin GS.The role of gastrin and cholecystokinin in normal and neoplastic gastrointestinal growth.J Gastroenterol Hepatol 1995;10:215-232.
20 Reubi JC,Schaer JC,Waser B.Cholecystokinin(CCK)-Aand CCK-B/gastrin receptors in human tumors.Cancer Res 1997;57:1377-1386.
21 Weinberg DS,Ruggeri B,Barber MT,Biswas S,Miknyocki S,Waldman SA.Cholecystokinin A and B receptors are differentially expressed in normal pancreas and pancreatic adenocarcinoma.J Clin Invest 1997;100:597-603.
22 Upp Jr JR,Singh P,Townsend Jr CM,Thompson JC.Predicting response to endocrine therapy in human pancreatic cancer with cholecystokinin receptors.Gastroenterology 1987;92:1677A.
23 Kano M,Shoda J,Satoh S,Kobayashi M,Matsuzaki Y,Abei M,et al.Increased expression of gallbladder cholecystokinin:a receptor in prairie dogs fed a high-cholesterol diet and its dissociation with decreased contractility in response to cholecystokinin.J Lab Clin Med 2002;139:285-294.
24 Karplus G,Ruiz R,Thomas DG,Ehrlich PF.Cholecystokinin receptor positivity in children with chronic acalculous gallbladder dysfunction:a pilot study to investigate the etiology of chronic acalculous gallbladder dysfunction.J Pediatr Surg 2008;43:850-853.
25 Sato N,Miyasaka K,Suzuki S,Kanai S,Ohta M,Kawanami T,et al.Lack of cholecystokinin-A receptor enhanced gallstone formation:a study in CCK-A receptor gene knockout mice.Dig Dis Sci 2003;48:1944-1947.
26 Miyasaka K,Takata Y,Funakoshi A.Association of cholecystokinin A receptor gene polymorphism with cholelithiasis and the molecular mechanisms of this polymorphism.J Gastroenterol 2002;37:102-106.
27 Okada N,Kubota A,Imamura T,Suwa H,Kawaguchi Y,Ohshio G,et al.Evaluation of cholecystokinin,gastrin,CCK-A receptor,and CCK-B/gastrin receptor gene expressions in gastric cancer.Cancer Lett 1996;106:257-262.
28 Clerc P,Dufresne M,Saillan C,Chastre E,André T,Escrieut C,et al.Differential expression of the CCK-A and CCK-B/gastrin receptor genes in human cancers of the esophagus,stomach and colon.Int J Cancer 1997;72:931-936.
29 Moonka R,Zhou W,Bell RH Jr.Cholecystokinin-A receptor messenger RNA expression in human pancreatic cancer.J Gastrointest Surg 1999;3:134-140.
30 Schulz S,Röcken C,Mawrin C,Schulz S.Immunohistochemical localization of CCK1 cholecystokinin receptors in normal and neoplastic human tissues.J Clin Endocrinol Metab 2005;90:6149-6155.
31 Lowenfels AB,Maisonneuve P,Boyle P,Zatonski WA.Epidemiology of gallbladder cancer.Hepatogastroenterology 1999;46:1529-1532.
32 Tewari M.Contribution of silent gallstones in gallbladder cancer.J Surg Oncol 2006;93:629-632.
33 Hur K,Kwak MK,Lee HJ,Park DJ,Lee HK,Lee HS,et al.Expression of gastrin and its receptor in human gastric cancer tissues.J Cancer Res Clin Oncol 2006;132:85-91.
