靶向对比剂CLT1-(Gd-DTPA)在小鼠乳腺癌磁共振分子成像效果的研究
2011-06-02FurongYeEunKeeJeongDenisParkerZhengRongLu
Furong Ye, Eun-Kee Jeong, Denis Parker, Zheng-Rong Lu
Introduction
Magnetic resonance imaging (MRI)is a powerful imaging modality for morphological and functional imaging. MRI provides anatomical images of soft tissues with high spatial resolution, but is often limited for molecular imaging because of its low sensitivity[1-3].Signif i cant efforts have been devoted to the design and development of effective targeted MRI contrast agents for molecular imaging of cancer biomarkers expressed on cancer cell surfaces in the last three decades.Targeting agents, e.g. peptides, antibodies and proteins,have been conjugated to polymers or nanoparticles containing a large number of Gd (III)chelates to increase local concentration of contrast agents and to generate detectable MR signals[4-8]. However, the targeted contrast agents based on these polymers or nanoparticles are too large to be excreted from the body via renal filtration,resulting in prolonged tissue retention. Long-term tissue accumulation of Gd (III)based contrast agents may release Gd (III)ions and cause toxic side effects such as systemic nephrogenic fi brosis[9,10]. An innovative design of safe and effective targeted MRI contrast agents is necessary for satisfying the unmet needs for MR cancer molecular imaging.
We have recently hypothesized that effective MR cancer molecular imaging can be achieved by targeting the molecular biomarkers with high expression in tumor stroma using the agents that can be readily excreted[11-13]. Tumor stroma has a unique extracellular matrix composed of cancer related biomacromolecules needed for cancer cell survival and proliferation. For example, fi brin and fibronectin in tumor stroma are known to associate with increased microvessel permeability and tumor angiogenesis in neoplastic tissues[14,15]. Fibrin and fi bronectin are highly expressed and form complexes in the mesh network of malignant tumors. Their complexes could be a suitable biomarker for cancer molecular imaging with MRI. We have recently synthesized and tested a CLT1-(Gd-DTPA)as a targeted MRI contrast agent for cancer molecular imaging[11]. CLT1 is a cyclic decapeptide, CGLIIQKNEC, that specifically binds to the fi brin-f i bronectin complexes in various tumor tissues with little non-specif i c binding to normal tissues[16]. Our initial study has shown that the agent is effective for MR cancer molecular imaging in a mouse colon cancer model. In this study, we further evaluated the eff i cacy of CLT1-(Gd-DTPA)for cancer molecular imaging in mice bearing MDA-BM-231 breast tumor xenografts.
1 Materials and Methods
1.1 Synthesis of CLT1-(Gd-DTPA)
The CLT1 peptide CGLIIQKNEC was first synthesized using standard solid-phase peptide synthesis from Fmoc-protected amino acids on a 2-chlorotrityl chloride resin. At the end of the peptide synthesis, an excess of DTPA dianhydride in DMSO was reacted with the peptide on the beads at room temperature for 4 hours to conjugate DTPA at the N-terminal of the peptide. The resin was completely washed with water,DMF, dichloromethane and methanol three times each.The CLT1-DTPA was then removed from the resin using a TFA solution (TFA 94%, 1, 2-ethanedithiol 2.5%,triisobutylsilane 2.5%, and water 1%). The product was exposed to air for about 2 hours to allow the formation of disulf i de bonds for the cyclic peptide and then purif i ed using preparative HPLC with a C18 column. CLT1-(Gd-DTPA)was fi nally prepared by complexation of CLT1-DTPA with Gd (OAc)3at pH 6. Excess Gd (OAc)3was removed by precipitation at pH 11. The final product was purif i ed by preparative HPLC.
1.2 Animal model
Human breast carcinoma cell line MDA-MB-231 was purchased from American Type Culture Collection(ATCC, Manassas, VA). The MDA-MB-231 human breast cancer cells were cultured in Leibovitz's L-15 medium with 2 mM L-glutamine and 10% FBS. Female athymic nu/nu mice (6 weeks old)were purchased from the National Cancer Institute (Frederick, MD). The mice were cared for according to the guidelines of the IACUC,University of Utah. The mice were subcutaneously implanted in both lower fl anks with 2×106MDA-MB-231 cells in a mixture of 50 μl culture media and 50 μl Matrigel. Mice were used for MRI study when tumor sizes reached 0.5-0.8 cm.
1.3 MR imaging
MRI study was performed on a Siemens Trio 3T scanner using a human wrist coil[17]. A clinical contrast agent, Gd(DTPA-BMA), was used as a control. A group of 3 mice were used for each contrast agent. The mice were anesthetized by intramuscular administration of a mixture of ketamine (45 mg/kg)and xylazine (6 mg/kg)for MRI. The CLT1-(Gd-DTPA)and Gd(DTPA-BMA)was intravenously injected at a dose of 0.05 and 0.1 mmol/kg, respectively. High resolution 3D images were acquired with a 3D FLASH sequence with 25°flip angle, TR/TE=7.8/2.7 ms, 0.5 mm slice thickness,120 mm field of view (FOV), 0.5×0.5×0.5 mm3voxel size. T1-weighted 2D axial tumor images were acquired with a 2D spin echo sequence with 90°flip angle, TR/TE = 400/10 ms, 2.0 mm slice thickness, 50 mm FOV, and 0.5×0.5×2 mm3voxel size. Contrast enhanced MR images were acquired before and at 1,5, 10, 15, 20, 30 and 60 minutes after injection. MR images were analyzed with Osirix (http://homepage.mac.com/rossetantoine/osirix/)software. The signal intensity was measured in the tumor periphery and inner core, and the signal to noise ratio (SNR)in the tumor tissues was calculated as SNR= (SItissue-SInoise)/SDnoise.MR signal intensity was also measured in the tissue of interest from high-resolution 3D images and SNR was calculated in these tissues. Statistical analysis was performed with Prism software (Version 4.0b, GraphPad software Inc., San Diego, CA)using two-way repeated ANOVA. Bonferroni post-test was used to determine the signif i cant difference in the comparisons among the conjugates. Statistical signif i cance was considered when P < 0.05.
1.4 Histological analysis
Immunohistochemistry was performed to evaluate the expression of fibronectin in tumor tissue.Mice bearing MDA-MB-231 tumor xenografts were sacrificed, and tumor tissues were removed and fixed with 3% paraformaldehyde and embedded in paraffin.Tumor tissue was sectioned into 4 μm slices and incubated in 3% hydrogen peroxide, 10% methanol for 10 min at room temperature to block endogenous peroxidase activity. The tumor sections were then boiled in antigen retrieval solution (1 mmol/L Tris-HCl, 0.1 mmol/L EDTA, pH=8.0)for 15 minutes at high power in a microwave and incubated with primary anti-f i bronectin antibody (Sigma-Aldrich, cat#F3648)at appropriate dilutions overnight. After washing with PBS buffer,the sections were incubated with biotinylated secondary antibody and a horseradish peroxidase-streptavidin complex for 1 h each. Tissue samples were then colorized with 3, 3' diaminobenzidine (DAB)substrate,counterstained, mounted and visualized with a brightfield microscope.
2 Results and Discussion
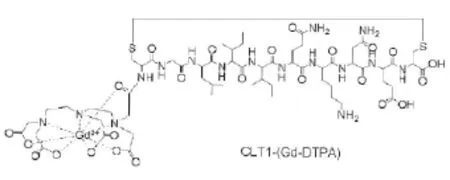
Figure 1 Chemical structure of CLT1-(Gd-DTPA)

Figure 2 T1-weighted 2D spin-echo images of mice bearing MDA-MB 231 xenografts before and at 5, 30 and 60 minutes after intravenous injection of CLT1-(Gd-DTPA)(A, 0.05 mmol/kg)and Omniscan® (B, 0.1 mmol/kg). Arrows point to the tumor.
The structure of CLT1-(Gd-DTPA)is shown in Figure 1. Gd-DTPA is a clinical MRI contrast agent.The cyclic peptide CLT1 was conjugated to one of the fi ve carboxylic groups of DTPA. The fi nal product had four carboxylates, one amide carbonyl group and three amino groups complexed to a Gd(III)ion. It should have a thermodynamic stability higher than the clinical agent,Gd(DTPA-BMA). T1 and T2 relaxivities of CLT1-(Gd-DTPA)were 4.22 and 4.45 mM-1sec-1at 3T, comparable to other Gd(III)based clinical MRI contrast agents.
The effectiveness of CLT1-(Gd-DTPA)for MR molecular imaging of fibrin-fibronectin complexes in tumor stroma was evaluated in female athymic nu/nu mice bearing MDA-MB-231 human breast carcinoma xenografts. Figures 2 shows the axial T1-weighted 2D spin-echo images of the tumor tissues of the mice bearing MDA-MB-231 tumor xenografts before and after the injection of CLT1-(Gd-DTPA)and Gd(DTPABMA). Significant enhancement was observed in tumor tissues for both agents in the fi rst 5 minutes postinjection. Gd(DTPA-BMA)was then cleared from the tumor tissue and tumor enhancement returned to the background level after 30 minutes post-injection.Strong enhancement was still visible in the tumor tissues at 60 minutes after injection for CLT1-(Gd-DTPA). The enhancement of the targeted agent in the tumor periphery was more significant than that in the tumor core. Figure 3 shows the signal-to-noise ratios(SNR)in the tumor periphery before and at various time points after injecting the contrast agents. The SNR in the tumor tissue with Gd(DTPA-BMA)reduced to the background level at 30 minutes post-injection, while approximately 30% increase of SNR was observed at 60 minutes after the injection in the tumor periphery with the targeted contrast agent. The SNR indirectly ref l ects the concentration of the agents in tumor, with higher SNR indicating higher concentrations of the contrast agents in the tissues. The results indicate the binding and retention of the targeted contrast agent in tumor tissue for signif i cant tumor enhancement.

Figure 3 The plots of SNR versus time in tumor periphery before and after injection of CLT1-(Gd-DTPA)(△)and Omniscan®(○).
MR signal intensity with the contrast agents in other regions of interest was also determined in the high-resolution 3D MR images of mice to preliminarily evaluate their biodistribution and pharmacokinetic properties. Figure 4 shows SNR in the blood, liver and muscle of the mice injected with CLT1-(Gd-DTPA)and Gd(DTPA-BMA). CLT1-(Gd-DTPA)had similar blood SNR kinetics as Gd(DTPA-BMA), indicating that the targeted agent had similar pharmacokinetics as the clinical agent with little binding to the soluble fi bronectin and fi brinogen in the blood. The blood SNR decreased rapidly for both agents and almost returned to the background level at 60 minutes after the injection.CLT1-(Gd-DTPA)had higher initial SNR in the liver than Gd(DTPA-BMA), possibly due to the lipophilic nature of the peptide. The SNR of the targeted agent in the liver then returned to the similar level as that of Gd(DTPA-BMA)at 60 minutes after the injection.Both agents resulted in minimally increased SNR in the muscle. The results suggest that CLT1-(Gd-DTPA)behaved as a low molecular weight contrast agent and had little non-specif i c binding to normal tissues.
Immunohistochemistry confirmed the presence of fibronectin in the MDA-MB-231 breast cancer xenografts after in vivo MR imaging. Figure 5 shows the histological images of fibronectin in MDA-MB-231 tumor tissues. The high expression of fibronectin was shown in the tumor stroma with staining of an antifibronectin antibody. The abundant presence of fibrinfibronectin complexes in tumor stroma allowed speci fic and prolonged binding of a sufficient amount of CLT1-(Gd-DTPA)to generate measurable enhancement in the tumor tissue.

Figure 4 Plots of SNR versus time in the blood (A), liver (B),and muscle (C)of mice bearing MDA-MB 231 xenografts before and after injection of CLT1-(Gd-DTPA)(△, 0.05 mmol/kg)and Omniscan® (○, 0.1 mmol/kg).
It is difficult for contrast enhanced MRI to effectively detect the biomarkers expressed on cancer cells because of its low sensitivity. We have shown that contrast enhanced MRI can be effective for molecular imaging of cancer biomarkers abundantly expressed in tumor stroma. The presence of cancerrelated biomacromolecules in tumor stroma facilitates cancer cell survival and promotes tumor proliferation and metastasis[14,15]. These biomacromolecules can be used as viable biomarkers for cancer diagnosis and prognosis. Fibrin and fi bronectin form clot complexes upon fibrin polymerization in tumor stroma and serve as a provisional matrix for adhesion and migration of cancer cells. Due to the abundant presence of the fibrinfibronectin complexes in the tumor stroma, a sufficient amount of CLT1-(Gd-DTPA)could specifically bind to the molecular targets. CLT1-(Gd-DTPA)had little non-specific binding to the proteins in the blood and normal tissue. Since the targeted contrast agent was a low molecular weight chelate, the unbound agent could readily be cleared from blood circulation and normal tissues. Consequently, significant tumor enhancement with little background enhancement was observed with the targeted agent since 30 minutes after the injection at a reduced dose. The low molecular weight targeted contrast agent is advantageous as compared to targeted macromolecular contrast agents for further clinical development due to rapid excretion and minimal retention in normal tissues.

Figure 5 Immunostaining of fibronectin in MDA-MB-231 breast tumor xenografts (right)and muscle tissue (right)with anti-fibronectin primary antibody. The arrow points to the fi bronectin in the extracellular space of tumor tissue.
CTL1-(Gd-DTPA)resulted in significant enhancement in the tumor periphery of the breast tumor tissue, the regions rich of angiogenic microvessels,similar to the enhancement in the colon cancer model reported in our previous publication[11]. It has been known that the presence of fibrin and fibronectin in tumor extracellular matrix might promote tumor angiogenesis[14,15]. Strong enhancement with the targeted contrast agent in tumor periphery suggested high expression of the fibrin-fibronectin complexes in the highly angiogenic regions of the tumor tissue. This result correlated well to the possible biological functions of fibrin-fibronectin complexes in cancer biology.Accurate characterization of tumor angiogenesis is critical for cancer diagnosis and prognosis and for assessment of tumor response to anticancer therapies.MRI with CLT1-(Gd-DTPA)has a potential to be used for characterizing angiogenesis in breast cancer and for non-invasive evaluation of the efficacy of antiangiogenesis therapy.
3 Conclusion
The targeted contrast agent CLT1-(Gd-DTPA)had minimal non-specific binding in blood and in normal tissues. A sufficient amount of CLT1-(Gd-DTPA)specifically bound in the breast tumor and generated strong and prolonged enhancement in the tumor tissue for effective molecular imaging of breast cancer with MRI. CLT1-(Gd-DTPA)is a promising low molecular weight targeted contrast agent for MR molecular imaging of the fibrin-fibronectin complexes in breast cancer. It has a great potential for the accurate detection and diagnosis of breast cancer.
4 Acknowledgements
This research was supported in part by the NIH R01 CA097465. We greatly appreciate Dr. Yongen Sun and Ms. Melody Johnson for their technical assistance in animal handling and MRI data acquisition.
[1]Stephen RM, Gillies RJ. Promise and progress for functional and molecular imaging of response to targeted therapies. Pharm Res, 2007, 24(6): 1172-1185.
[2]Caravan P, Ellison JJ, McMurry TJ, et al. Gadolinium(III)Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem Rev, 1999, 99(9): 2293-2352.
[3]Göhr-Rosenthal S, Schmitt-Willich H, Ebert W, et al.The demonstration of human tumors on nude mice using gadolinium-labelled monoclonal antibodies for magnetic resonance imaging. Invest Radiol, 1993, 28(9): 789-795.
[4]Sipkins DA, Cheresh DA, Kazemi MR, et al. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med, 1998, 4(5): 623-626.
[5]Curtet C, Maton F, Havet T, et al. Polylysine-Gd-DTPAn and polylysine-Gd-DOTAn coupled to anti-CEA F(ab')2 fragments as potential immunocontrast agents. Relaxometry,biodistribution, and magnetic resonance imaging in nude mice grafted with human colorectal carcinoma. Invest Radiol, 1998, 33(10):752-761.
[6]Ke T, Jeong EK, Wang X, et al. RGD targeted poly(L-glutamic acid)-cystamine-(Gd-DO3A)conjugate for detecting angiogenesis biomarker alpha(v)beta3 integrin with MRT, mapping. Int J Nanomedicine, 2007, 2(2):191-199.
[7]Flacke S, Fischer S, Scott MJ, et al. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation, 2001, 104(11):1280-1285.
[8]Amirbekian V, Lipinski MJ, Briley-Saebo KC, et al.Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A, 2007, 104(3):961-966.
[9]Ersoy H, Rybicki FJ. Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J Magn Reson Imaging,2007, 26(5): 1190-1197.
[10]Sieber MA, Pietsch H, Walter J, et al. A preclinical study to investigate the development of nephrogenic systemic fibrosis: a possible role for gadolinium-based contrast media. Nvest Radiol, 2008, 43(1): 65-75.
[11]Ye F, Wu X, Jeong EK, Jia Z, et al. A peptide targeted contrast agent specif i c to fi brin-f i bronectin complexes for cancer molecular imaging with MRI. Bioconjug Chem,2008n 19(12):2300-2343.
[12]Tan M, Wu X, Jeong EK, et al. Peptide-targeted Nanoglobular Gd-DOTA monoamide conjugates for magnetic resonance cancer molecular imaging.Biomacromolecules, 2010, 11(3):754-761.
[13]Tan M, Wu X, Jeong EK, et al. An effective targeted nanoglobular manganese(II)chelate conjugate for magnetic resonance molecular imaging of tumor extracellular matrix.Mol Pharm, 2010, 7(4): 936-943.
[14]Dvorak HF, Senger DR, Dvorak AM, et al. Regulation of extravascular coagulation by microvascular permeability.Science, 1985, 227(4690): 1059-1061.
[15]Neri D, Carnemolla B, Nissim A, et al. Targeting by affinity-matured recombinant antibody fragments of an angiogenesis associated fibronectin isoform. Nat Biotechnol, 1997, 15(12): 1271-1275.
[16]Pilch J, Brown DM, Komatsu M, et al. Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. Proc Natl Acad Sci U S A, 2006, 103(8):2800-2804.
[17]Zong Y, Guo J, Ke T, et al. Effect of size and charge on pharmacokinetics and in vivo MRI contrast enhancement of biodegradable polydisulfide Gd(III)complexes. J Control Release, 2006, 112(3): 350-356.
