Isolation and characterization of Hainantoxin-II, a new neurotoxic peptide from the Chinese bird spider (Haplopelma hainanum)
2010-12-25PANJianYiYUZhiQiang
PAN Jian-Yi, YU Zhi-Qiang
(1. School of Life Sciences, Zhejiang Sci-Tech University, Hangzhou 310018, China;2. Faculty of Pharmaceutical Sciences, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki 852-8521, Japan)
Isolation and characterization of Hainantoxin-II, a new neurotoxic peptide from the Chinese bird spider (Haplopelma hainanum)
PAN Jian-Yi1,*, YU Zhi-Qiang2
(1.School of Life Sciences,Zhejiang Sci-Tech University, Hangzhou310018,China;2.Faculty of Pharmaceutical Sciences,Graduate School of Biomedical Sciences,Nagasaki University,Nagasaki852-8521,Japan)
Hainantoxin-II (HnTx-II), a novel neurotoxin, was isolated from the venom of the Chinese bird spider(Haplopelma hainanum) by cation exchange chromatography and reverse-phase HPLC. The toxin was a single chain polypeptide with calculated molecular weight of 4 253.135 obtained by mass spectrometry. The complete amino acid sequence of HnTx-II was determined by Edman degradation and found to contain 37 residues with three disulfide bonds.Results showed HnTx-II can reversibly paralyze cockroaches for several hours after intra-abdominal injection with ED50of 16 μg/g and kill the insects immediately at a dose of 60 μg/g. It was also shown to kill mice at a LD50value of 1.41μg/g after intracerebroventricular injection. Hainantoxin-II shares 91% sequence homology with Huwentoxin-II (HwTx-II), an insecticidal peptide from another bird spider (Haplopelma schmidti) with a unique scaffold. While HnTx-II and HwTx-II both exhibit toxic activities in insects and mammals, HnTx-II shows higher insecticidal activity and lower lethiferous activity of mammals than HwTx-II. These results help clarify structural-functional relationships of the polypeptide toxin.
HnTx-II; Neurotoxin; Insecticidal peptide; Spider venom;Haplopelma hainanum
Spider venom is a source of natural products with diverse biological activities on both insects and mammals (Rash & Hodgson, 2002), and are of interest as potential pharmacological tools (Ashok et al, 2000;Fletcher et al, 1997; Liang et al, 1993), drug discovery structures (Yan & Michael, 1998), and determinants of the role and diversity of neuronal ion channels (Escoubas,2006). Theraphosidaes, commonly called tarantulas orbird spiders, are among the best known of all spider species (Ashok, 2000). Two Chinese bird spiders,Haplopelma hainanumandHaplopelma schmidti(Ornithoctonus huwenum), have been identified as new species distributed in the hilly areas of southern China and generally inhabiting holes underground. They have a similar morphology, and both kill insects and small vertebrates for food. The crude venom ofH. schmidtipossesses diverse compounds, such as neurotoxin (Liang,2004), lectin (Li & Liang, 1999; Liang & Lin, 2000), and serine protease inhibitor (Liang, 2004), which cause numerous biological activities in insects and mammals.The structure and function of toxic peptides fromH.schmidtihave been investigated intensively over the last ten years (Liang, 2004). In addition, some components ofH. hainanumvenom have been found to be toxic to mice,and act as tetrodotoxin-sensitive sodium current inhibitors (Xiao & Liang, 2003a) or channel inhibitors(Xiao & Liang, 2003b; Li et al, 2004).
In this study, we report on the isolation, amino acid sequence, and biological activities of a novel toxic peptide, Hainantoxin-II (HnTx-II), from the venom ofH.hainanum. This peptide shares high sequence homology with Huwentoxin-II (HwTx-II), a peptide fromH.schmidtiwith unique disulfide bridge linkage (Shu et al,2001 & 2002). While both these neurotoxins affect mammals and insects, HnTx-II shows higher insecticidal activity and lower lethiferous activity in mammals than that of HwTx-II. The findings from this study may help provide new insights into structural-functional relationships of the polypeptide toxin.
1 Materials and Methods
1.1 Venom and animals
AdultH. hainanumspiders were collected in the hilly area of Tongshi County, Hainan Province in southern China. The venom was obtained by electrical stimulation of the spider's fangs (Qu et al, 1997), and was immediately lyophilized and stored at −20℃.Kunming albino mice were obtained from Hunan Medical University and cockroaches (Periplaneta americana) from Peking University.
1.2 Chemicals
Reagents for N-terminal sequencing were obtained from Applied Biosystems (USA). The chromatographic solvent acetonitrile (ACN) was purchased from Linhai Chemicals (China), HPLC grade. Trifluoroacetic acid(TFA) was purchased from Sigma Chemical Company(USA). All other reagents were of analytical grade.
1.3 Purification of venom fraction
The lyophilized venom was dissolved in double-distilled water (1 mg/mL) and was centrifuged at 8000 r/min for 15 min. The supernatant was then subjected to cation-exchange chromatography on a 10 mm × 100 mm column initially equilibrated with buffer A containing 0.02 mol/L sodium phosphate buffer, pH 6.5. The column was eluted with a 0−45% linear gradient of buffer B (1 mol/L sodium chloride, 0.02 mol/L sodium phosphate, pH 6.5) over 50 min at a flow rate of 1.0 mL/min. Elution of peptide was monitored at 280 nm.Fraction B from ion-exchange chromatography was further subjected to reverse-phase HPLC on a 3.9 mm ×300 mm C18 column initially equilibrated with 0.1%trifluoroacetic acid in water (buffer A). Elution was performed with a 0−45% linear gradient of buffer B(acetonitrile containing 0.1% trifluoroacetic acid) over 60 min at a flow rate of 0.7 mL/min. Elution of the fraction was monitored at 280 nm. Elution with retention time of 25 min was further fractionated by reverse-phase HPLC at the same conditions as described above.
1.4 Mass spectrometry analysis
Peptide molecular masses were determined by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) with a delayed extraction ion source equipped with a nitrogen laser of 337 nm. The acceleration voltage was set to 20 kV. The matrix used was α-cyano-4-hydroxy-cinnamic acid(CCA, saturated solution in 50% ACN: 0.1% TFA, 1:1).
1.5 Carboxymethylation of peptide and N-terminal sequencing
Peptide reduction and S-carboxymethylation was performed as reported previously (Zhang et al, 1993).The carboxymethylated and native peptide were determined by an Applied Biosystems Model 491 gas-phase Pro-sequencer (ProciseTM) according to the Edman degradation procedure.
1.6 Biological activity
The biological activities of HnTx-II were examined by injecting it into cockroaches and mice. Intraabdominal injections of HnTx-II in 0.9% NaCl solution into mature male cockroaches (BW 0.5 g) (Shu et al,1999) and intra-abdominal and intracerebroventricular injections into Kunming albino mice (BW 18 − 22 g)were performed (Chen et al, 1995). Mice phrenic neuromuscular transmission preparations were used for pharmacological experiments (Liang et al, 1998).
2 Results
2.1 Purification and characterization of HnTx-II
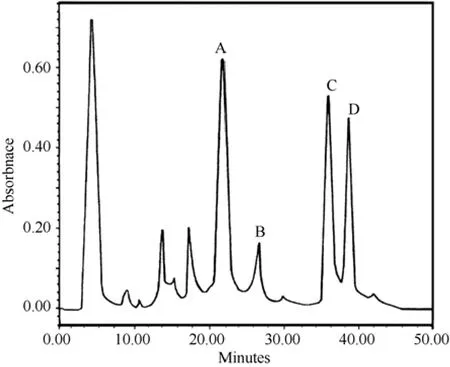
Fig. 1 Cation-exchange HPLC of crude H. hainanum venom
The cation-exchange chromatography elution profile of unsexedH. hainanumcrude venom is shown in Fig.1. Several peaks were observed, which demonstrate that crude venom was relatively complex. Peak B in Fig.1 was collected and further purified by subjection to reverse-phase HPLC, and two peaks with retention time of about 25 and 35 min, respectively, were found (Fig.2A). The peak with 25 min retention time was found to be toxic to insects and was fractionated again by reverse-phase HPLC. The elution showed one sharp and symmetric peak (Fig. 2B), designated to HnTx-II.MALDI-TOF mass spectrometry was applied to analyze the molecular weight of native and cysteines carboxymethylation of HnTx-II. The observed molecular masses were 4253.383 (Fig.3A) and 4601.338 (Fig.3B),respectively, which indicated that the toxin contained six cysteines and formed three disulfide bonds. The amino acid sequence was determined by automated Edman degradation of the S-carboxymethylated samples. The intact native toxin was also sequenced for conformation.The complete amino acid sequence obtained was LFECS VSCEI EKEGN KDCKK KKCKG GWKCK FNMCV KV. The theoretical molecular mass calculated from the sequence was 4 259.135 and the mass was in good agreement with mass spectrometry data.Homologous peptides were searched and HnTx-II was found to share high homology with HwTx-II, an insecticidal peptide and mammalian neurotoxin from the Chinese bird spiderHaplopelma schmidti(Selenocosmia huwena) (Shu et al, 1999), ESTx1 from the tarantulaAphonopelmaCalifornicum(Eurypelma Californicum)(Savel-Niemann, 1989), and TXP1 from the Mexican red knee tarantulaBrachypelma smithii(Kaiser et al, 1994).Their sequence alignment and identities are shown in Tab. 1.
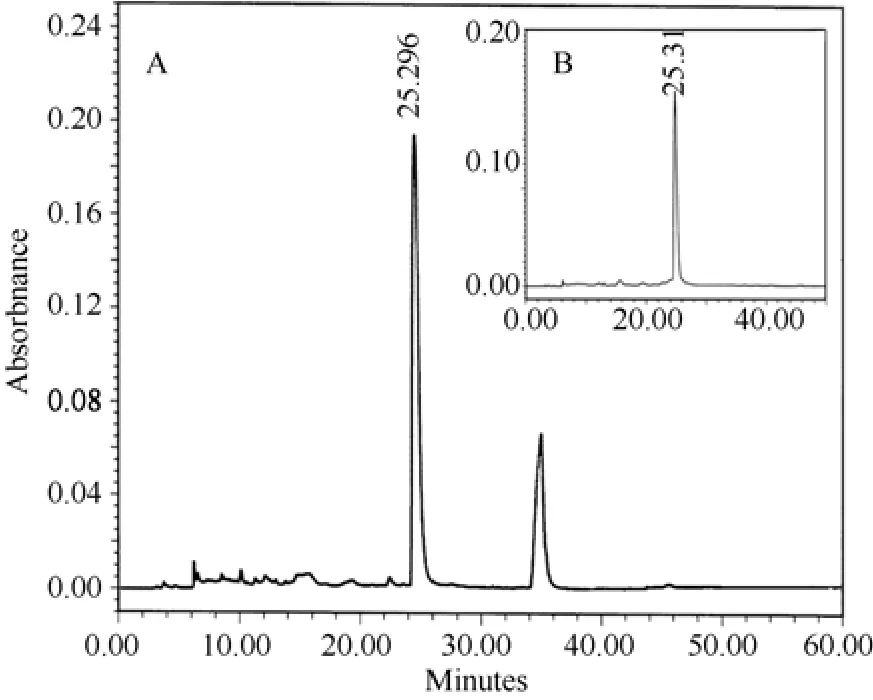
Fig. 2 Purification of HnTx-II by reverse-phase HPLC
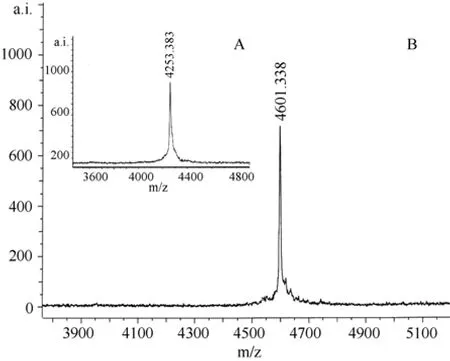
Fig. 3 MALDI-TOF mass spectra of the HnTx-II
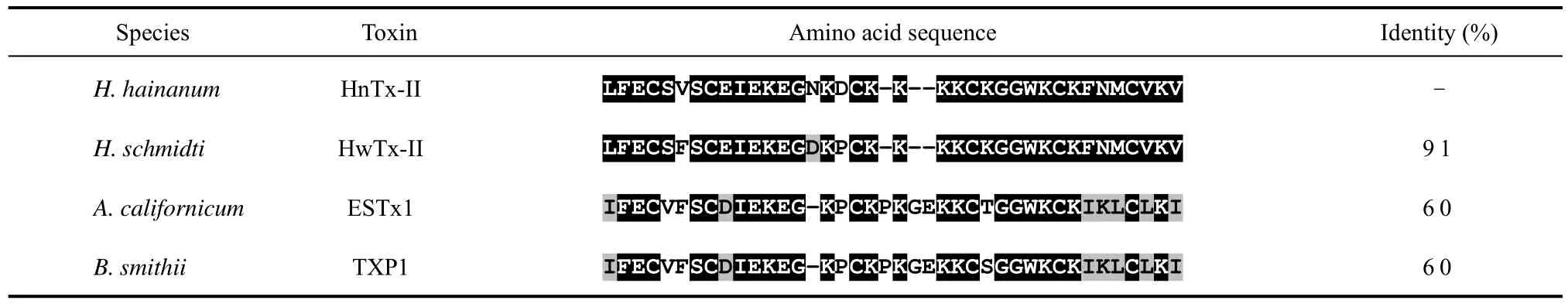
Tab. 1 Sequence alignment of HnTx-II, HwTx-II, ESTx1 and TXP1
2.2 Biological activity assays
HnTx-II is a multi-activity peptide both in insects and mammals, likely due to its similar amino acid sequence to HwTx-II. Cockroaches and Kunming albino mice were used to verify HnTx-II’s biological activity.Flexible cockroaches were quickly paralyzed after intra-abdominal injection of 20 μg/g HnTx-II compared to the control insects injected with non-toxic peptide,H.schmidtilectin-I (SHL-I). The paralytic effect lasted over 10 hours. Larger doses (40 μg/g body weight) killed cockroaches half a day after the injection, while increasing the dosage to 60 μg/g body weight, killed the cockroaches within a few minutes. The dose required to paralyzed half of the insects (ED50) was 16 μg/g (Tab. 2).
No visible symptoms or behavior change were observed, however, after intra-abdominal injection of 50 μg/g HnTx-II into Kunming albino mice. Pharmacological experiments, which were carried out by using isolated mice phrenic nerve-diaphragm preparations treated with HnTx-II at 50 μg/mL, showed that HnTx-II could not block neuromuscular transmission (figure not shown) but could kill mice after intracerebroventricular injection(LD50value of 1.41 μg/g) (Tab. 2). From these results,we inferred that HnTx-II was an insecticidal peptide that affected the central nervous system of mammals.

Tab. 2 The biological activities of HnTx-II and HwTx-II
3 Discussion
The Chinese bird spiderH. hainanumis a newly identified species and its venom has been found to contain a mixture of components. The main component,HnTx-I, (Fig.1A), had no effect on mice and cockroaches after intra-abdominal injection (Li et al, 2003). The other two components, HnTx-III and HnTx-IV (Fig.1C and Fig.1D, respectively), are novel mammalian nerve sodium channel blockers (Xiao & Liang, 2003b). In our study, HnTx-II showed 91% sequence identity with HwTx-II (Tab. 1), and their cysteine residues were highly conserved. HwTx-II has a unique disulfide linkage pattern (C1-C3, C2-C5 and C4-C6) (Fig.4) (Shu et al,2001), which differs from other spider neurotoxin peptides with an inhibitor cystine knot (ICK) motif(Pallaghy et al, 1994; Norton & Pallaghy, 1998). The three-dimensional structure of HwTx-II is also the first representative of a novel structural scaffold in peptide toxins (Shu et al, 2002). The unique disulfide linkage pattern and structural scaffold of HwTx-II should be valid for HnTx-II owing to its high sequence homology and conserved cysteine residues with HwTx-II.

Fig. 4 The unique disulfide linkage pattern of HWTX-Ⅱ(C1-C3, C2-C5 and C4-C6)
Biological activity assays showed that HnTx-II was an insect neurotoxin with an ED50value of 16 μg/g,which was much lower than that observed for HwTx-II(127 μg/g) (Shu et al, 1999) (Tab. 2). This indicates that HnTx-II has higher insecticidal activity than HwTx-II.Despite its weak insecticidal activity, HwTx-II also blocked neuromuscular transmission in mice of the phrenic nerve diaphragm preparations within 91 minutes at a concentration of 50 μg/mL (Shu et al, 1999), while HnTx-II had no effect on neuromuscular transmission even at a dose of 500 μg/mL (ten times higher than that of HwTx-II) (Tab. 2). Additionally, though both toxins killed mice after intra-cerebroventricular injection, their LD50values showed a significant difference with the value of HnTx-II being about five times higher than that of HwTx-II (Tab. 2). That is, HnTx-II had lower mammalian neurotoxin activity than HwTx-II.
The roles played by protein or peptide depend on their three-dimensional structures (i.e., based on amino acid composition). The bioactivity variations between HnTx-II and HwTx-II depend, therefore, upon the three amino acid residues mutated at position 6 (Val→Phe), 15(Asn→Asp), and 16 (Asp→Pro) of the sequences (Tab.1). Additionally, HnTx-II is a single chain peptide with a calculated molecular mass of 4253, while HwTx-II formed by two subunits of molecular mass 4284 and 4300,respectively (Shu et al, 1999). Further investigation of the variations in chemical structure and biological activity between HnTx-II and HwTx-II should provide insight into the proteins’ structure-function relationships, and lead to synthesis or expression of HnTx-II mutants with strong insecticidal activity to serve as useful agricultural drugs.
Ashok RB, Sasaki T, Gopalakrishnakone P, Kazuki S, Manjunatha RK,Bay BH. 2000. Purification, structure determination and synthesis of covalitoxin-II, a shot insect-specific neurotoxic peptide from the venom of theCoremiocnemis validus(Singapore tarantula) [J].FEBS Lett, 474: 208-212.
Chen WY, Li BH, Wu HY. 1995. Inhibition of defecation caused by morepinephrine and intracerebroventricular injection of methoxamine in mice [J].Chn J Pharmacl Toxic, 9: 277-279.
Escoubas P. 2006. Molecular diversification in spider venoms: A web of combinatorial peptide libraries [J].Mol Divers, 10: 545-554.
Fletcher JI, Smith R, O'Donoghue SI, Nilges M, Connor M, Howden ME, Christie MJ, King GF. 1997. The structure of a novel insecticidal neurotoxin, omega-atracotoxin-HV1, from the venom of an Australian funnel web spider [J].Nat Struct Biol, 4: 559-566.Kaiser I I, Griffin PR, Aird SD, Hudiburg S, Shabanowits J, Francis B,John TR, Hunt DF, Odell GV. 1994. Primary structures of two proteins from the venom of the Mexican red knee tarantula(Brachypelma smithii) [J].Toxicon,32: 1083-1093.
Li D, Xiao Y, Hu W, Xie J, Bosmans F, Tytgat J, Liang S. 2003.Function and solution structure of hainantoxin-I, a novel insect sodium channel inhibitor from the Chinese bird spiderSelenocosmia hainana[J].FEBS Lett, 555: 616-622
Li D, Xiao Y, Xu X, Xiong X, Lu S, Liu Z, Zhu Q, Wang M, Gu X,Liang S. 2004. Structure-activity relationships of hainantoxin-IV and structure determination of active and inactive sodium channel blockers [J].J Biol Chem, 279: 37734-37740.
Li F, Liang SP. 1999. Assignment of the three disulfide bonds ofSelenocosmia huwenalectin-I from the venom of the spiderSelenocosmia huwena[J].Peptides,20: 1027-1034.
Liang S. 2004. An overview of peptide toxins from the venom of the Chinese bird spiderSelenocosmia huwenaWang [=Ornithoctonus huwena(Wang)] [J].Toxicon, 43: 575-585.
Liang SP, Lin L. 2000. Haemagglutination activity analysis ofSelenocosmia huwenalectin-I from the venom of the Chinese bird spiderSelenocosmia huwena[J].Chn J Biochem Mol Biol, 16:92-95.
Liang SP, Peng XJ, Huang RH, Chen P. 1999. Biochemical identification ofSelenocosmia hainanasp. Nov. from South China(Araneae, Theraphoside) [J].Life Sci Res, 3: 299-303.
Liang SP, Zhang DY, Pan P, Chen Q, Zhou PA. 1993. Properties and amino acid sequence of Huwentoxin-I, a neurotoxin purified from the venom of the Chinese bird spiderSelenocosmia Huwena[J].Toxicon, 31: 969-978.
Norton RS, Pallaghy PK. 1998. The cystine knot structure of ion channel toxins and related polypeptides [J].Toxicon, 36: 1573-1583.Pallaghy PK, Nielsen KJ, Craik DJ, Norton RS. 1994. A common structural motif incorporating a cystine knot and a triple-stranded β-sheet in toxic and inhibitory polypeptides [J].Prot Sci, 3:1833-1839.
Qu Y, Liang S, Ding J, Liu X, Zhang R, Gu X. 1997. Proton nuclear magnetic resonance studies on Huwentoxin-I from the venom of the spiderSelenocosmia huwena: 2. Three-dimensional structure in solution [J].J Pro Chem, 16: 565-574.
Rash DL, Hodgson WC. 2002. Pharmacology and biochemistry of spider venoms [J].Toxicon, 40: 225-254.
Savel-Niemann A. 1989. Tarantula (Eurypelma californicum) venom, a multicomponent system [J].Biol Chem Hopp Seyler, 370:485-498.
Shu Q, Huang RH, Liang SP. 2001. Assignment of the disulfide bonds of Huwentoxin-II by Edman degradation sequencing and stepwise thiol modification [J].Eur J Biochem, 268: 2301-2307.
Shu Q, Liang SP. 1999. Purification and characterization of Huwentoxin-II, a neurotoxin peptide from the venom of the Chinese bird spiderSelenocosmia huwena[J].J Peptide Res, 53:486-491.
Shu Q, Lu SY, Gu XC, Liang SP. 2002. The structure of spider toxin Huwentoxin-II with unique disulfide linkage: evidence for structure evolution [J].Prot Sci, 11: 245-252.
Xiao YC, Liang SP. 2003a. Purification and characterization of Hainantoxin-V, a tetrodotoxin-sensitive sodium channel inhibitor from the venom of the spiderSelenocosmia hainana[J].Toxicon,41(6): 643-650.
XiaoYC, Liang SP. 2003b. Inhibition of neuronal tetrodotoxin-sensitive Na+channels by two spider toxins: hainantoxin-III and hainantoxin-IV [J].Euro J Pharmac, 477: 1-7.
Yan LZ, Michael EA. 1998. Lycotoxins, antimicrobial peptides from venom of the wolf spiderLycosa carolinensis[J].J Biol Chem,273: 2059-2066.
Hainantoxin-Ⅱ的分离纯化及其结构与功能分析
潘建义1,*, 喻志强2
(1. 浙江理工大学 生命科学学院, 杭州 310018; 2. 长崎大学 药学院生物医学研究生院, 长崎 日本 852-8521)
从分布于我国海南省的海南捕鸟蛛 (Haplopelma hainanum) 毒素中, 利用阳离子交换色谱和反相高效液相色谱分离得到一种多肽神经毒素, 命名为Hainantoxin-Ⅱ(HnTx-Ⅱ)。MALDI-TOF质谱分析表明该多肽毒素相对分子质量为4 253.135, 其氨基酸序列经Edman降解测序为LFECS VSCEI EKEGN KDCKK KKCKG GWKCK FNMCV KV, 其中的6个Cys形成3对二硫键。同源性搜索表明, HnTx-Ⅱ与Huwentoxin-Ⅱ(HwTx-Ⅱ)的同源性高达91%, 仅有3个氨基酸残基不同。HwTx-Ⅱ为从虎纹捕鸟蛛中提取的杀虫肽, 该多肽具有独特的结构模体。活性分析表明HnTx-Ⅱ与HwTx-Ⅱ具有相似的生物学活性。经腹腔注射HnTx-Ⅱ, 美洲蜚蠊可被麻痹, 其ED50为16 μg/g;而当剂量增加到60 μg/g时, 可立即杀死美洲蜚蠊, 其杀死昆虫活性明显强于HwTx-Ⅱ。此外, HnTx-Ⅱ经脑室注射可杀死小鼠, 其LD50为1.41 μg/g, 该活性却明显低于HwTx-Ⅱ。这两种多肽毒素的结构与功能的差异为进一步阐明多肽毒素的结构与功能之间的关系提供良好的研究模型。
HnTx-Ⅱ; 神经毒素; 杀虫肽; 蜘蛛毒素; 海南捕鸟蛛
Q959.226; Q956; Q51
A
0254-5853(2010)06-0570-05
2010-06-04; 接受日期:2010-10-19
浙江省教育厅科研项目(Y200805989)
date: 2010-06-04; Accepted date: 2010-10-19
s: This work was supported by the Research Project of the Education Department of Zhejiang Province, China (Y200805989)*
(通讯作者), E-mail: panjy@zstu.edu.cn
猜你喜欢
杂志排行
Zoological Research的其它文章
- Shape change in viable eggs of the collembolan Folsomia candida provides insight into the role of Wolbachia endosymbionts
- Digestive enzyme and alkaline phosphatase activities during the early stages of Silurus soldatovi development
- Morphological changes of silver and bighead carp in the Yangtze River over the past 50 years
- Positive influence of traditional culture and socioeconomic activity on conservation: A case study from the black-and-white snub-nosed monkey (Rhinopithecus bieti) in Tibet
- Bats and marsupials as indicators of endemism in the Yungas forest of Argentina
- A new record of Dasyatid fish in China: Dasyatis laosensis
