分光光度法间接测定电镀废水中的氨基磺酸
2010-11-16DEEPANAGARAJABALASUBRAMANIANGEORGE
DEEPA B, NAGARAJA K S, BALASUBRAMANIAN N, GEORGE M
分光光度法间接测定电镀废水中的氨基磺酸
DEEPA B, NAGARAJA K S, BALASUBRAMANIAN N, GEORGE M
提出了一种利用已知过量的亚硝酸盐与氨基磺酸反应,对氨基磺酸进行分光光度测定的方法。未反应的亚硝酸根采用其与对硝基苯胺及 N–(1–萘基)乙二胺二盐酸盐(NEDA)之间的重氮偶合反应来测定。所生成的偶氮染料的最大吸收波长为545 nm,实测摩尔吸光度为6.1 × 104L/(mol·cm)。该方法可用于测量总体积为25 mL的样品中0 ~ 25 μg的氨基磺酸。当样本数为 10时,测定 15 μg氨基磺酸的相对标准偏差为2%。将该方法用于测定镍、铅、铁、钌、铟等金属的氨基磺酸盐电镀液废水中氨基磺酸的含量,其结果采用加标回收率方法进行了验证。
氨基磺酸;重氮偶合;分光光度法;电镀废水
1 Introduction
Sulfamic acid has a unique combination of properties that makes it particularly well suited for scale removal and chemical cleaning operations. Salts of sulfamic acid are used in electro plating and electroforming operations as well as for manufacturing flameretardants and weed killers[1]. Because sulfamate salts are highly soluble, higher concentration of metal ions can be attained which permit higher rates of plating than sulfate baths[2]. Sulfamates used in plating solutions include the salts of nickel, cobalt, cadmium, iron, ruthenium, palladium, indium and lead. The nickel deposits from sulfamate baths are harder and less ductile with low internal stresses as compared to deposits from additive free Watt’s bath.
Nickel deposits from sulfamate bath are considerably stronger at cryogenic temperature than deposit from Watt’s bath[3]. Nickel sulfamate baths are usually employed to produce deposits for engineering purposes with the control of mechanical properties such as stress, ductility and hardness. Sulfamate baths are primarily used in industrial nickel plating and electroforming where low internal stress of the deposit, high plating rates are of practical importance.
Besides being reactive, sulfamic acid and its salts are corrosive to eyes, skin and respiratory track. Inhalation may cause pulmonary edema, which can be delayed for several hours and there is a risk of death in serious cases[4]. The exposure limit of sulfamic acid according to American Conference of Governmental Industrial Hygiene (ACGIH) was found to be 10 mg/m3and Occupational Safety and Health Administration (OSHA) was found to be 15 mg/m3(total) and 5 mg/m3(resp). Hence the determination of residual amounts of sulfamic acid in the effluents of sulfamate baths is essential.
There are reports available in the literature for the determination of sulfamic acid by techniques as varied as gravimetry[5], gasometry[6-7]and titrimetry[8]. The recent spectrophotometric method reported for the trace determination of sulfamic acid involves acid hydrolysis of sulfamic acid to form ammonium sulfate. The formed ammonium sulfate was determined based on Berthelot reaction[9]. In this study, sulfamic acid is indirectly determined by a facile and sensitive spectrophotometric method based on diazo-coupling reaction.
2 Experimental
All absorbance measurements were made using Elico SL177 scanning spectrophotometer with 1 cm glass cells.
2. 1 Reagents
All chemicals used were analytical grade reagents and distilled water was used in their preparations.
Standard sulfamic acid solution (1 000 ppm) was prepared by diluting 0.100 0 g of sulfamic acid in 100 mL of distilled water. Working standards of 100 ppm and 5 ppm were prepared by dilution.
Nitrite solution (1 000 ppm) was prepared by diluting 0.150 0 g of sodium nitrite in 100 mL of distilled water. A suitable aliquot of this solution was diluted to get 15 ppm of nitrite.
p-Nitroaniline (0.05%) was prepared by dissolving 0.05 g of the reagent in 100 mL of 1:1 hydrochloric acid. N-(1-naphthyl)ethylenediamine dihydrochloride [NEDA] (0.1%) was prepared by dissolving 0.1 g in water and diluting it to 100 mL.
Sulfuric acid (2 N) was prepared by diluting 36 N concentrated sulfuric acid (specific gravity: 1.84) with distilled water.
Formic acid solution (9 800 ppm) was prepared by diluting 1 mL of formic acid (98%) in 100 mL of distilled water. A suitable aliquot of this solution was diluted to get 2 000 ppm of formic acid.
Bromine water (saturated).
2. 2 Calibration graph
In each of a series of 25 mL-calibrated flasks was placed 1 mL of 15 ppm of nitrite solution, followed by acidification with 7.5 mL of 2 N sulfuric acid. After the addition of 5 mL of sample containing 0-25 g of sulfamic acid, the solution was allowed to stand for 20 min, followed by the addition of 1 mL of 0.05% p-nitroaniline and 1 mL of 0.1% NEDA, and diluted to 25 mL. All absorbance measurements were made at 545 nm against water using 1 cm glass cells.
3 Results and discussion
Diazo coupling method is extensively used for the determination of nitrite[10]. The decomposition of nitrite in acid medium in the presence of sulfamic acid[11]is a well-known reaction.
In this work, a fixed concentration of nitrite was allowed to react with varying concentration of sulfamic acid in acid medium. The unreacted nitrite is determined by diazo coupling reaction involving p-nitroaniline and NEDA to form the azo dye, 4-(4-nitrophenylazo)-N-(1-napthyl)ethylenediamine dihydrochloride (Figure 1).

Figure 1 Reaction scheme图1 反应历程
Sulfamic acid, when added in increasing amounts to fixed concentrations of nitrite destroys nitrite and consequently there is a concomitant fall in the unreacted nitrite. This is observed as a proportional decrease in the absorbance of the azo dye formed as compared to the dye formed with the blank, which is not treated with sulfamic acid.
The various parameters involved in the formation of the azo dye were optimized. It was found that 15 μg/mL nitrite was sufficient to produce an absorbance value of 0.72 ± 0.01 in the presence of 7.5 mL of 2 N sulfuric acid, 1 mL of 0.05% p-nitroaniline and 1 mL of 0.1% NEDA solution. For the determination of sulfamic acid, 15 μg/mL of nitrite was treated with 5 mL of sample containing 25 μg of sulfamic in the presence of 7.5 mL of 2 N sulfuric acid and allowed to stand for 5-20 min. After the expiry of the standing time the unreacted nitrite was determined by diazo coupling reaction. The absorbance of the azo dye formed by the unreacted nitrite was found to be minimum and constant for the standing period 15 min and 20 min. Hence a standing period of 20 min was allowed for the decomposition of nitrite.
Two blanks were prepared for this system. The reagent blank, which contained optimum concentrations of all the reagents except sulfamic acid, gave maximum absorbance at 545 nm (Figure 2). The other blank was prepared in the absence of nitrite and sulfamic acid, to determine the contribution of the other reagents to the absorbance of the system at 545 nm. As the absorbance of the second blank at 545 nm was comparable to that of water, the absorbance of the developed dye was measured against water. The decreasing absorbance values at 545 nm were plotted against the increasing concentration of sulfamic acid to obtain the calibration graph with a negative slope. The calibration graph was linear in the concentration range 0-25 μg of sulfamic acid in an overall aqueous volume of 25 mL. The equation of the line is Y = -0.022 9X + 0.720 6 where Y is the absorbance and X (μg) is the amount of sulfamic acid.
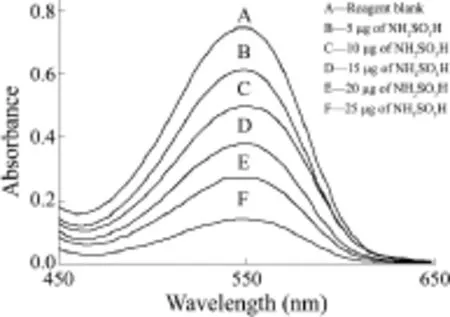
Figure 2 Absorbance spectra measured against water图2 以水为参比时测得的吸收光谱
The absorbance spectrum of the system is shown in Figure 2 and the calculated molar absorptivity was found to be 6.1 × 104L/(mol·cm) at 545 nm. The developed color was stable for 3 h. The precision of the method was established at the 15 μg level of sulfamic acid and the relative standard deviation (RSD) was found to be 2% (n = 10). The correlation coefficient of the calibration plot was calculated to be -0.999 9, confirming a linear decrease in absorbance with increasing concentration of sulfamic acid. The detection limit of sulfamic acid in the proposed method is 2 g.
3. 1 Interference Study
The effect of various cations and anions usually associated with sulfamate plating baths were studied at 15 μg level of sulfamic acid. A variation in the absorbance of the solution of more than ±0.01 absorbance, in the presence of any co-existing matrix constituent was taken as an indication of interference. The results are given in Table 1. Common metal ions usually associated with sulfamate baths such as Ni2+, Pb2+, Pd2+, In3+, Ru4+and commonly used additives such as boric acid, ammonium ion, triethanolamine (TEA), dextrose and nitrate do not interfere in the determination at 1 000 μg level. Fe2+interferes in the determination at 10 µg level. The interference up to 1 000 μg was overcome by the excess addition of saturated bromine water (indicated by the pale yellow color) to the sample solution prior to the addition of nitrite to oxidize Fe2+to Fe3+. The excess bromine color was removed by the addition of 1 mL of 2 000 ppm formic acid.
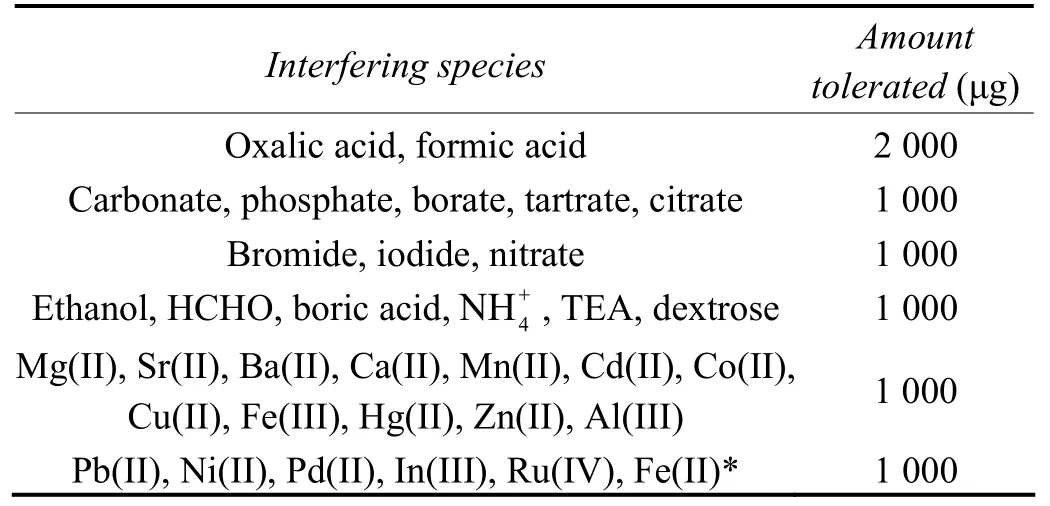
Table 1 Effects of interfering species on the determination of sulfamic acid at 15 μg level表1 测定15 μg氨基磺酸时各种干扰物质的影响
3. 2 Application
The suitability of the method for the determination of sulfamic acid in the effluents of sulfamate bath solutions was evaluated. As effluents samples were not readily available samples were prepared by dilution of synthetic metal sulfamate bath solutions prepared following the procedures recommended by Lowenheim[2]and metal finishing guide book and directory[3]. The compositions of plating baths are given in Table 2. The raw wastewater streams from respective electroplating baths contain nickel[12]in the concentration range 0.019-2 954 mg/L, iron in the concentration range 0.06-1 482 mg/L and lead in the concentration range 0.663-25.39 mg/L. The bath solutions were diluted to get the nickel, iron and lead concentrations as 3 000 mg/L, 1 500 mg/L and 30 mg/L respectively. For indium value is not available and it is diluted to 1 500 mg/L like that of iron. In the case of precious metal wastes very low concentration of metal ions are found and they are returned to refineries for the recovery of metals. In the case of ruthenium-containing wastewater no value is reported and the value is adjusted similar to the value of platinum[11]reported as 0.112-6.46 mg/L. For the experiment the solution was diluted to have a concentration of 6.5 mg/L of Ruthenium. The resulting sample solutions were suitably diluted to have sulfamic acid concentration of not more than 25 μg in a maximum volume of 5 mL. For analysis, 2 mL aliquots of suitably diluted synthetic sulfamate bath effluents were taken and overall volume was adjusted to 5 mL using distilled water. The determination was completed following the procedure described under the calibration graph. The results obtained are tabulated in Table 3. The values obtained were validated by standard addition and recovery studies of added sulfamic acid. The observed results show quantitative recovery of added sulfamic acid.
4 Conclusion
A new simple, indirect sensitive spectrophotometric method is proposed for the determination of sulfamic acid in the concentration range of 0-25 μg in a sample volume of 5 mL. The molar absorptivity is 6.1 × 104L/(mol·cm) and the correlation coefficient was found to be -0.999 9. The relative standard deviation is 2% for n = 10 at 15 μg levels of sulfamic acid. The salient features of the developed method are high sensitivity, practically free from interferences and simplicity for routine analysis of sulfamic acid in plating bath effluents.
[1] Kirk-Othmer Encyclopedia of Chemical Technology [M]. 4th ed. New York: John Wiley and Sons, 1997: 23, 120.
[2] LOWENHEIM F A. Modern Electroplating [M]. 3rd ed. New York: JohnWiley and Sons, 1974: 249.

Table 2 Compositions of sulfamate plating baths表2 氨基磺酸盐镀液的组成
[3] Metal Finishing 53rdGuidebook and Directory [M]. Hackensack: Metals and Plastics Publications Inc., 1985: 177, 256, 258, 335.
[4] POHANISH R P, GREENE S A. Hazardous Chemical Safety Guide for the Plastic Industry [M]. New York: McGraw-Hill Inc., 2000.
[5] BAUMGARTEN P, KRUMMACHER A-H. Über die umsetzung von schwefeltrioxyd mit ammoniak in wäßriger zusammensetzung der sog.„schwefeltrioxyd-nebel” [J]. Berichte der deutschen chemischen Gesellschaft, 1934, 67 (7): 1257-1260.
[6] MEUWESEN A, MERKEL H. Gasvolumetrische bestimmung der amidosulfonsäure, H2NSO3H [J]. Zeitschrift für anorganische und allgemeine Chemie, 1940, 244 (1): 89-93.
[7] CARSON W N. Gasometric determination of nitrite and sulfamate [J]. Analytical Chemistry, 1951, 23 (7): 1016-1019.
[8] BOWLER W W, ARNOLD E A. Comparison of methods for determination of sulfamates [J]. Analytical Chemistry, 1947, 19 (5): 336-337.
[9] DEEPA B, NAGARAJA K S, BALASUBRAMANIAN N. Spectrophotometric determination of sulfamic acid in metal sulfamate bath effluents [J]. Plating and Surface Finishing, 2005, 92 (4): 54-57.
[10] PANDURANGAPPA M, BALASUBRAMANIAN N. Extractive spectrophotometric determination of trace amounts of nitrogen dioxide, nitrite, and nitrate [J]. Mikrochimica Acta, 1996, 124 (1/2): 137-146.
[11] VOGEL A I. Vogel’s Textbook of Quantitative Inorganic Analysis [M]. BASSETT J, DENNY R C, JEFFERY C H, et al. 4th ed. London: ELBS, 1989: 515.
[12] SITTIG M. Electroplating and Related Metal Finishing: Pollutant and Toxic Materials Control [M]. Park Ridge: Noyes Data Corporation, 1978: 150.
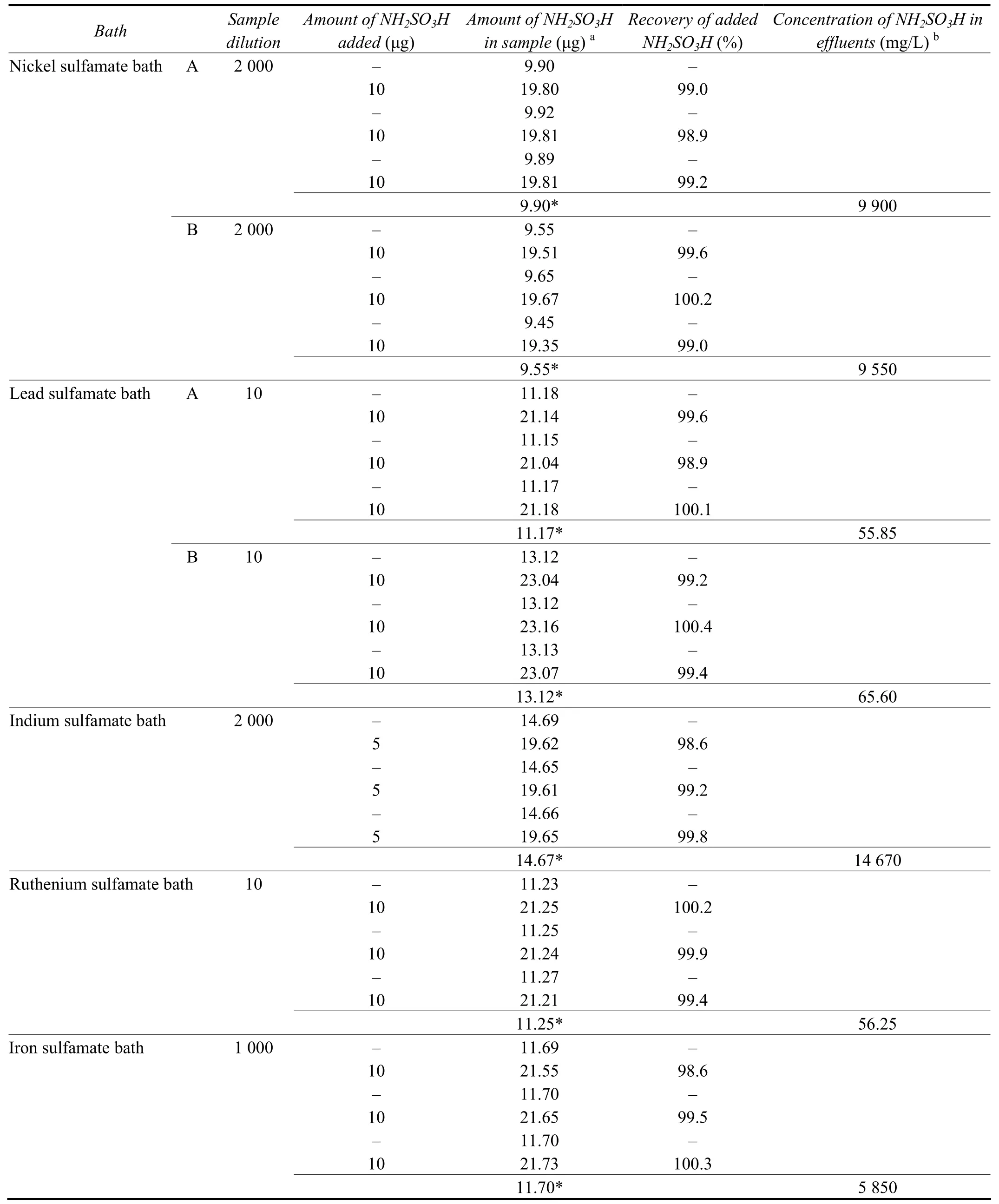
Table 3 Determination of sulfamic acid in different sulfamate bath effluents表3 不同氨基磺酸盐镀液废水中氨基磺酸的测定结果
Indirect spectrophotometric determination of sulfamic acid in electroplating effluents
B. Deepa1,*, K. S. Nagaraja1, N. Balasubramanian2, Mary George3,**
(1. Department of Chemistry, Loyola Institute of Frontier Energy (LIFE), Loyola College, Chennai-600 034, India; 2. Department of Chemistry, Indian Institute of Technology, Chennai-600 036, India; 3. Department of Chemistry, Stella Maris College, Chennai-600 086, India)
A proposed spectrophotometric method for the determination of sulfamic acid involves reaction of sulfamic acid with known excess of nitrite. The unreacted nitrite is determined by diazo coupling reaction involving p-nitroaniline and N-(1-naphthyl)ethylenediamine dihydrochloride [NEDA]. The absorption maximum (λmax) for the formed azo dye is at 545 nm and the calculated molar absorptivity is 6.1 × 104L/(mol·cm). The method is useful for determining sulfamic acid in the range of 0-25 μg in an overall volume of 25 mL, and the relative standard deviation is 2% for n = 10 at 15 μg level of sulfamic acid. The developed method was applied to the determination of sulfamic acid in the effluents of nickel, lead, iron, ruthenium and indium sulfamate electroplating baths. The results obtained were validated by recovery studies of added sulfamic acid.
sulfamic acid; diazo coupling; spectrophotometry; electroplating effluent
TQ153.12
A
1004 – 227X (2010) 11 – 0046 – 05
date:2010–06–01
s:Dr. B. Deepa, (E-mail) deepa_anal@yahoo.co.in; Dr. M. George, (E-mail) lavusha@yahoo.com.
Biography: Dr.B. Deepa obtained her Masters and Doctorate degree from Madras University. Her research area of interest lies in the development of novel analytical methods for determination of toxic water pollutants. She has to her credit five articles in internationally refereed journals. Presently she is a registered Indian patent attorney dealing with Intellectual property rights.
[ 编辑:温靖邦 ]
