犬传染性肝炎DNA疫苗安全性评价
2010-09-09郭瑞敏郑龙封志岚张焕铃连伟光刘福英王俊霞
郭瑞敏,郑龙,封志岚,张焕铃,连伟光,刘福英,王俊霞
(河北医科大学分子生物学研究室,河北省实验动物重点实验室,石家庄,050017)
研究报告
犬传染性肝炎DNA疫苗安全性评价
郭瑞敏,郑龙,封志岚,张焕铃,连伟光,刘福英,王俊霞
(河北医科大学分子生物学研究室,河北省实验动物重点实验室,石家庄,050017)
目的研究犬传染性肝炎核酸疫苗pVAX1-CpG-Loop的安全性。方法BALB/c小鼠随机分为4组,高剂量组(肌内注射每只200 μg)、低剂量组(肌内注射每只100 μg)、联合免疫组(肌内注射每只100 μg,皮下注射50 μg,滴鼻每只50 μg)和PBS组,每两周免疫1次,共免疫3次。末次免疫后4周、6个月检测血常规和血液生化及对F1代的影响,用PCR和RT-PCR的方法检测DNA疫苗的生物学分布和存留时间,末次免疫后4周和6个月取脏器观察病理损伤。结果各剂量组的主要血液学检测指标、对F1代的影响差异无显著性。末次免疫后4周各剂量组AST明显高于对照组。DNA疫苗在注射部位可存留8周,其中高剂量组和低剂量组在肝、脾、肾和注射部位有分布,联合免疫组在肺组织也有分布。末次免疫后4周小鼠肝肾有淋巴细胞浸润,6个月后慢性炎症明显好转。结论由犬传染性肝炎病毒DNA疫苗引起的肝肾损伤是一过性的,并且pVAX1-CpG-Loop没有整合到宿主基因组,也没有传递给F1代。
pVAX1-CpG-Loop;核酸疫苗;安全性评价;犬传染性肝炎
犬传染性肝炎(infectious canine hepatitis,ICH)是由犬传染性肝炎病毒引起的一种常见的急性败血性传染病[1]。疫苗已经成为预防疾病的主要策略。传统疫苗难以激活细胞毒性T淋巴细胞而诱导细胞免疫[2,3],而DNA疫苗能够诱导宿主产生特异性细胞免疫和体液免疫应答,从而达到预防和治疗疾病的目的[4,5]。本室已经构建的抗ICHV核酸疫苗pVAX1-CpG-Loop,免疫BALB/c小鼠后,诱导出特异性体液免疫和细胞免疫,产生杀伤表达病毒抗原靶细胞的CTL效应,显示出良好的免疫原性和免疫反应性[6]。本课题用抗犬传染性肝炎病毒核酸疫苗pVAX1-CpG-Loop免疫小鼠,对其安全性进行评价。
DNA疫苗的安全性主要考虑的一个方面是质粒DNA是否被整合到宿主细胞基因组[7-10]。整合的DNA质粒可能通过插入激活致癌基因或灭活抑癌基因而导致肿瘤的发生[11],如果在生殖细胞发生这种整合将会导致物种的改变,美国FDA规定任何一种基因治疗必须评估其潜在的整合到宿主生殖细胞的可能性以免引起物种的改变[12,13]。
1 材料与方法
1.1 实验动物
清洁级BALB/c小鼠112只,雌雄各半,来源于河北省实验动物中心【SCXK(冀)2003-1-003】,并按实验动物使用的3R原则给予人道的关怀。
1.2 试剂
Trizol,M-MLV,Taq酶购自天根生化科技有限公司。
1.3 PCR引物
Loop上游引物P1:5′-CGGGATCCATGAACTG CCTATTTAATGGATCAGGT-3′,
下游引物P2:5′-CCGCTCGAGGCGGCCATTGC CAAGCAGCT-3′;
β-actin上游引物:5′-GTGGGCCGCTCTAGG CACCAA-3′,
下游引物:5′-CTCTTTGATGTC ACGCACGATTT C-3′。
1.4 DNA疫苗
本室已完成犬肝炎病毒DNA疫苗pVAX1-CpG-Loop的构建。该DNA疫苗的提取为经典的质粒提取方法并经过纯化。用Nano Drop ND-1000 DNA核酸蛋白定量测定仪,检测其浓度和纯度。使用前用PBS稀释至所需浓度。
1.5 动物分组与免疫
BALB/c小鼠随机分为4组,高剂量组(每只每次200 μg),低剂量组(每只每次100 μg),联合免疫组(每只每次200 μg)和PBS组。高、低剂量组和PBS组为股四头肌注射,每两周免疫1次共免疫3次,免疫体积均为每只100 μL。联合免疫组为肌内注射每只每次100 μg,皮下注射每只每次50 μg,滴鼻每只每次50 μg,每两周免疫1次,共3次,浓度为1 μg/μL。
1.6 观察指标
1.6.1 健康状况:每天观察并记录受试动物的一般活动及精神状况,粪、尿颜色及粪便是否成形等;如有死亡,记录死亡数并作解剖观察。每周称量体重1次,计算出每组动物的平均体重,观察体重增长情况。
1.6.2 血液检测:末次免疫后4周,眼眶取血,测定各组动物的血常规。末次免疫后4周和6个月时测定各组动物血液的丙氨酸转氨酶(ALT)和天冬氨酸转氨酶(AST)。
1.7 质粒pVAX1-CpG-Loop在小鼠体内的分布及存留时间
1.7.1 RT-PCR:提取末次免疫后7、15、30 d动物肝、脾、心脏、肾、肺、卵巢,睾丸及注射部位肌肉的RNA,反转录【引物oligo(dT)】后PCR扩增;PCR反应条件:94℃预变性5min;94℃45 s,58℃(β-actin: 50℃)45 s,72℃1 m in,共35个循环,72℃10 m in。1%琼脂糖凝胶电泳鉴定,凝胶成像分析仪分析。
1.7.2 PCR:提取末次免疫后1周、8周、12周动物的肝、脾、心脏、肾、肺、卵巢、睾丸及注射部位肌肉的DNA,进行PCR扩增。反应条件:94℃预变性5 m in;94℃45 s,58℃45 s,72℃1 min,共35个循环,72℃10 m in。1%琼脂糖凝胶电泳鉴定,凝胶成像分析仪分析。
1.8 对F1代的影响
末次免疫4周后,将相同剂量组的雌、雄鼠配对,每组7对,观察其受孕情况,幼崽的生长状况。提取出生2周幼崽的肝、脾DNA并进行PCR扩增观察质粒DNA整合情况。
1.9 组织病理观察
末次免疫4周和6个月后分别取各剂量组动物肝、脾、心脏、肾、肺及注射部位的肌肉进行HE染色观察有无组织损伤。
2 结果
2.1 健康状况
在受试过程中未发现动物死亡。各实验组与对照组动物活动、精神状态、尿及粪便性状未见明显差异。各受试动物体重增长情况没有明显差异(P>0.05)(图1)。

注:受试动物免疫7周后的体重改变,数据为每组动物(4只/组)体重的均数±标准差。图1免疫后受试动物的体重改变Note:Vaccinated mice and control mice were weighed weekly for 7 weeks post vaccination.The data are expressed as the mean±SD of 4 mice per group.Fig.1 The changes of body weight following vaccination.
2.2 血液检测
受试动物血常规各监测指标在各组间均无明显差异。末次免疫后4周和6个月后丙氨酸转氨酶(ALT)在各组动物无明显差别,高剂量组、低剂量组、联合免疫组的天冬氨酸转氨酶(AST)在末次免疫后4周均高于对照组(P<0.05)。末次免疫6个月后各组与对照组相比,AST和ALT差异均无显著性(P>0.05,表1)。

表1 免疫4周后血液生化分析Tab.1 Blood biochemistry analysis at 4 weeks after vaccination
2.3 质粒pVAX1-CpG-Loop在小鼠体内的分布及存留时间
受试动物注射pVAX1-CpG-Loop或PBS后,在上述各时间点分别提取各组动物脏器RNA和DNA,用RT-PCR和PCR的方法检测结果显示:末次免疫第7天肝、脾和肌肉中均有Loop的表达,而15d后Loop基因仅在肝和肌肉中表达,30d在所有脏器中均不表达(图2)。分别于末次免疫第1周、第8周、第12周提取各脏器的基因组DNA,用PCR方法扩增Loop基因,第1周高剂量和低剂量组肝、脾、肾、肌肉和联合免疫组肝、脾、肾、肺和肌肉均有分布;第8周各剂量组仅在肌肉有分布;第12周各脏器均无分布。PBS组各脏器未发现Loop基因的表达(图3)。

图2 Loop基因在受试动物体内的分布Fig.2 Tissue distribution of loop message.
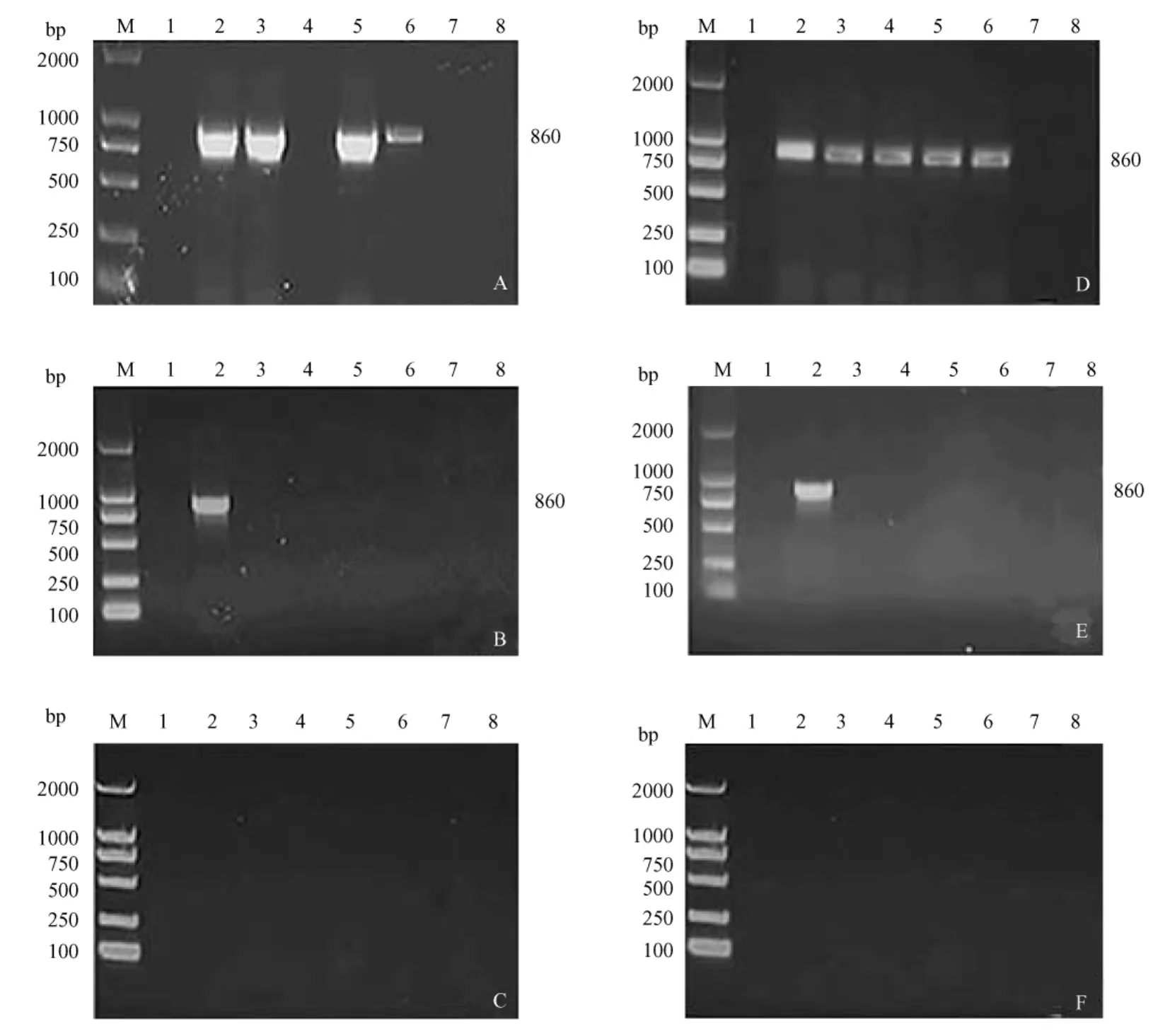
注:A、B、C分别为高剂量组在末次免疫BALB/c小鼠后1周、8周和12周质粒DNA在体内的分布,D、E、F分别为联合免疫组在上述各时间点其在体内的分布。图3质粒DNA在各组织内的分布和存留时间Note:Total cellular DNA obtained from each tissue was pooled and PCR was performed.PCR analysis of cellular DNA collected from BALB/c mice after immunization with high-dose vaccine at 1(A),8(B)and 12 weeks(C)post immunization.PCR amplification of the cellular DNA obtained from BALB/c mice immunized at multiple sites at 1(D),8 (E)and 12 weeks post vaccination(F).Lane 1:gonads;Lane 2:muscle;Lane 3:kidney;Lane 4:lung;Lane 5: sp leen;Lane 6:liver;Lane 7:heart;Lane 8:negative control.Fig.3 The distribution and persistence of p lasmid DNA in various tissues
2.4 对F1代影响
末次免疫4周后将相同注射剂量组的雌、雄鼠配对,生产后,计数幼崽的个数,各实验组与空白组没有显著差异。幼崽生长状况良好,出生时的体重各实验组与空白组没有显著差异。幼崽出生2周分别提取各剂量组肝、脾DNA并进行PCR,未发现有扩增产物,说明其没有整合到子代DNA中。
2.5 组织病理观察
结果表明,末次免疫后4周和6个月各受试动物脾、心脏、肺、注射部位的肌肉无明显损伤。各剂量组与PBS组相比,末次免疫4周后肝脏均有淋巴细胞等炎细胞浸润,出现水样变性,半年后上述病变明显减轻;末次免疫4周后各剂量组肾小球有明显淋巴细胞浸润,6个月后炎症明显缓解(图4,彩插7)。
3 讨论
DNA疫苗的安全性是疫苗在进入临床实验之前首先应考虑的问题,主要是确定其是否被整合到宿主基因组DNA中[14-16]。本研究采用PCR和RTPCR两种方法从DNA水平和RNA水平研究了质粒pVAX1-CpG-Loop在小鼠体内的分布和存留。βactin作为内参保证了RT-PCR系统的正确性。结果表明pVAX1-CpG-Loop在宿主体内的分布与疫苗免疫途径和注射剂量大小有关。末次免疫12周后被检组织没有检测到pVAX1-CpG-Loop DNA,说明质粒DNA没有整合到宿主基因组DNA中。
pVAX1-CpG-Loop免疫后,没有发现受试动物有明显的临床和血常规的异常变化。慢性炎症和AST升高提示疫苗引起了肝细胞损伤;据报道与ALT相比,AST分子量小,由于血浆半衰期短,肝细胞损伤时AST水平变化早,恢复快[17],本实验观察到pVAX1-CpG-Loop免疫小鼠后,血液AST 4个月时升高,6个月恢复到正常水平。核酸疫苗引起的免疫复合物沉积性肝损伤在Zi等[18]的研究中有报道,分析原因可能是由质粒DNA的长期存在和持续表达引起。但未见质粒引起肝AST升高的报道。
目前已有一些DNA疫苗被批准进入临床实验,但没有质粒整合到生殖系细胞DNA中的报道,多途径免疫小鼠,结果显示在被免疫小鼠的F1代体内没有发现Loop基因,表明质粒pVAX1-CpG-Loop没有整合到生殖细胞DNA中。
总之,本研究表明抗犬传染性肝炎病毒DNA疫苗免疫BALB/c小鼠产生肝、肾损伤,但随免疫时间的延长而逐渐恢复。并且质粒pVAX1-CpG-Loop没有整合到宿主基因组,也没有传递给F1代。
(本文图4见彩插7。)
[1]Tribe GW,Wolff DC.Protection of dogs against canine hepatitis with Toronto A26-61 virus(kennel cough isolate)hexon antigen[J].J Small Anim Pract,1973,14:251-255.
[2]Fishman B,Scarnell J.Persistence of protection after vaccination against infectious canine hepatitis virus(CAV-1)[J].Vet Rec,1976,99:509.
[3]Miller AS,Curtis R,Furminger IG.Persistence of immunity to infectious canine hepatitis using a killed vaccine[J].Vet Rec,1980,106:343-344.
[4]Loehr BI,Pontarollo R,Rankin R,et al.Priming by DNA immunization augments T-cell responses induced by modified live bovine herpesvirus vaccine[J].J Gen Virol,2001,82:3035-3043.
[5]Fuller DH,Rajakumar PA,W ilson LA,,et al.Induction of mucosal protection against primary,heterologous simian immunodeficiency virus by a DNA vaccine[J].J Virol,2002,76:3309-3317.
[6]Wang JX,Zheng L,Song SX,et al.Augmented humoral and cellular immune responses induced by canine adenovirus type 1 DNA vaccine in BALB/c mice[J].Viral Immunol,2007,20 (3):461-468.
[7]Wolff JA,Malone RW,Williams P,et al.Direct gene transfer into mouse muscle in vivo[J].Science,1990,247:1465-1468.
[8]Wurm FM,Petropoulos CJ.Plasmid integration,amplification and cytogenetics in CHO cells:questions and comments[J]. Biologicals,1994,22:95-102
[9]Martin T,Parker SE,Hedstrom R,et al.Plasmid DNA malaria vaccine:the potential for genomic integration after intramuscular injection[J].Hum Gene Ther,1999,10(5):759-768.
[10]Ledwith BJ,Manam S,Troilo PJ,et al.Plasmid DNA vaccines: investigation of integration into host cellular DNA following intramuscular injection in mice[J].Intervirology,2000,43(4-6):258-272.
[11]Nichols WW,Ledwith BJ,Manam SV,et al.Potential DNA vaccine integration into host cell genome[R].Ann NY Acad Sci,1995,772:30-39.
[12]Kang KK,Cho SM,Choi JH,et al.Safety evaluation of GX-12,a New HIV therapeutic vaccine:investigation of integration into the host genome and expression in the reproductive organs[J]. Intervirology,2003,46:270-276.
[13]Manam S,Ledwith BJ,Barnum AB,et al.Plasmid DNA vaccines:tissue distribution and effects of DNA sequence,adjuvants and delivery method on integration into host DNA[J]. Intervirology,2000,43:273-281.
[14]Tuomela M,Malm M,Wallen M,et al.Biodistribution and general safety of a naked DNA p lasmid,GTU-MultiHIV,in a rat,using a quantitative PCR method[J].Vaccine,2005,23: 890-899.
[15]W illiams RS,Johnston SA,Riedy M,et al.Introduction of foreign genes into tissues of living mice by DNA-coated micro projectiles[J].Proc Natl Acad Sci U S A,1991,88(7):2726-2730.
[16]Chun S,Daheshia M,Lee S,et al.Distribution fate and mechanism of immune modulation following mucosal delivery of p lasmid DNA encoding IL-10[J].J Immunol,1999,165(5): 2393-2402.
[17]张秀明,李建斋,魏明竟,等.现代临床生化检验学[M].第一版.北京:人民军医出版社,2001,169-170.
[18]Zi XY,Yao YC,Zhu HY,et al.Long-term persistence of hepatitis B surface antigen and antibody induced by DNA-mediated immunization results in liver and kidney lesions in mice[J].Eur J Immunol.2006,36:875-886.
Safety Evaluation of DNA Vaccine pVAX1-CpG-Loop against In fectious Canine Hepatitis Virus
GUO Rui-min,ZHENG Long,FENG Zhi-lan,ZHANG Huan-ling,LIAN Wei-guang,LIU Fu-ying,WANG Jun-xia
(Department of Molecular Biology,Hebei Key Lab of Laboratory Animals.Hebei Medical University,Shijiazhuang 050017,China)
Objective To evaluate the safety of a DNA vaccine against infectious canine hepatitis virus.M ethods Mice were divided into high dose group(DNA vaccine 200 μg/mouse,i.m),low dose group(DNA vaccine 100 μg each,i.m),multiple-site immunization group(intramuscular injection 100 μg,hypoderm ic injection s.c 50 μg,nasal instillation 50 μg)as well as control group(PBS 200 μl each,i.m).Treatment groups were vaccinated(or injected with PBS)every two weeks for 3 times.The hematology and the influence to generation F1 of mice were examined at 4 weeks after the last vaccination.Biochemistry of blood including AST and ALT was observed at 4 weeks and half year after the last immunization,respectively.PCR and RT-PCR were carried out to determine the biodistribution and persistence of the tested vaccine,respectively.Pathological observation was performed to evaluate if there were pathological alterations in the organs of mice.ResultsNo significant differences were observed between the vaccination and control groups by hematological examination,and the influence to generation F1 was not observed either.Compared with the control,there were conspicuous differences in AST among all the tested mice after 4 weeks,but it recovered half year later.The DNA vaccine can persist in the muscle tissue for 12 weeks,which was much longer than that in other tissues such as liver,spleen,kidney as well as lung.Leukomonocytes were observed in liver and spleen tissues of the immunized m ice.The chronic inflammation was recovered after half a year.ConlusionThe results of this study show that the alterations in the liver and kidney caused by the DNA vaccine against infectious canine hepatitis virus are temporal and that the pVAX1-CpG-Loop plasm id is not integrated into host tissues indefinitely nor transmissible to F1 progeny.
pVAX1-CpG-Loop;DNA vaccine;Safety evaluation;Infectious canine hepatitis
R392.3
A
1005-4847(2010)04-0345-05
2009-06-22
河北省自然基金(project No:C2006000816)。
郭瑞敏(1980-)女,硕士研究生,从事生物化学与分子生物学科研工作。
王俊霞,女,教授,硕士研究生导师。电话:031186261022
