Neuronal differentiation effects of vascular endothelial factor on bone marrow stromal cells*☆
2010-07-19LiYiQiaoyunLiuJinlingHanJingYeFangtingZhangGuanghuiCuiZhuqingZhou
Li Yi, Qiaoyun Liu, , Jinling Han, , Jing Ye, Fangting Zhang, Guanghui Cui, Zhuqing Zhou,
1Department of Neurology, Peking University Shenzhen Hospital, Shenzhen 518036, Guangdong Province, China
2Medical College of Shantou University, Shantou 515041, Guangdong Province, China
3Central Laboratory, Peking University Shenzhen Hospital, Shenzhen 518036, Guangdong Province, China
4Laboratory of Male Reproductive Medicine, Peking University Shenzhen Hospital, Shenzhen 518036, Guangdong Province, China
lNTRODUCTlON
Bone marrow stromal cells (BMSCs) have been demonstrated to differentiate into neural lineages[1]through the use of in vitro agents, such as chemical inducers[2], cytokines[3-5], co-culture with neural cells[6-7],cytokine gene transfection[8], and the combination of chemical inducers and cytokines[9-10]. However, very few inducers have been used for clinical application.
Vascular endothelial growth factor (VEGF)is a 34-48 kD dimer composed of disulfide-linked subunits, each with the same NH2-terminal amino acid sequence, which was originally isolated as an endothelial mitogen and a potent enhancer of vascular permeability[11]. It has been demonstrated to function as an angiogenic factor and a neuroprotective growth factor that promotes neurogenesis[12]. Many studies have shown the neurotrophic functions of VEGF.
For instance, VEGF plasmid treatment significantly reduces infarct volume and enhances striatal neurogenesis in the adult rat brain following stroke[13]. Moreover,VEGF enhances maturation in stroke-induced cortical neurogenesis and dendritic formation of newborn neurons in adult mammalian brains[14]. However, in vitro neuronal differentiation effects of BMSCs, through the use of VEGF induction,remain uncertain.
The present study analyzed the possibility of in vitro neuronal differentiation from BMSCs induced by VEGF.
MATERlALS AND METHODS
Design
In vitro comparative study.
Time and setting
This study was performed at the Central Laboratory and Laboratory of Male Reproductive Medicine, Shenzhen Hospital of Peking University from October 2008 to August 2009.
Materials
Healthy, adult, Sprague Dawley rats, weighing 200–300 g,were provided by the Laboratory Animal Center of Medical Department in Guangdong Province (No. SCXK(Yue) 2008-0002). All experimental procedures were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of the People’s Republic of China[15].
Main reagents and instruments used are as follows:

?
Methods
Isolation and culture of BMSCs
BMSCs were isolated from the tibia and femur of adult Sprague Dawley rats. The bone ends were cut, and the bone marrow was rinsed using a syringe filled with medium. The solution was collected in a centrifuge tube,gently triturated, and centrifuged at 1 200 r/min for 6 minutes. The supernatant was discarded, and the cells were resuspended in 10 mL DMEM/F12 supplemented with 10% FBS, 2 mmol/L L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin, seeded into two 25-cm2plastic flasks, and incubated with 5% CO2at 37 °C for 72 hours. The free-floating cells were discarded, and the medium was replaced. The entire volume of medium was replaced every 3–4 days for 2 weeks. When the cultures approached con fl uency, the cells were treated with 0.25% trypsin-EDTA for 1–2 minutes at room temperature, followed by additional culture at a ratio of 1: 2 per passage. The third passage of undifferentiated BMSCs were harvested for the following experiments.
BMSCs identified by flow cytometry
The hematopoietic cell marker CD45 and the stem cell marker CD90 were detected by flow cytometry[16]to identify BMSCs. The BMSCs were detached from the culture flasks using 0.25% trypsin-EDTA. The cells were then washed twice with 0.01 mol/L phosphate-buffered saline (PBS) and suspended with 0.01 mol/L PBS (106cells/100 μL). The cells were incubated with phycoerythrin-labeled mouse anti-rat CD45 (0.25 μg/106cells)and FITC-labeled mouse anti-rat CD90 (1 μg/106cells)for 30 minutes at 4 °C. After two washes with PBS, fluorescence-activated cell sorting analysis was performed using a Caliber cytometer (EpcisXL).
In vitro neuronal differentiation
The third passage BMSCs (5 × 103cells/mL) were seeded onto coverslips in two 6-well culture plates, which contained DMEM/F12 supplemented with 10% FBS,2 mmol/L L-glutamine, 100 IU/mL penicillin and 100 μg/mL streptomycin for 24 hours. The media was replaced with pre-induction media containing DMEM/F12,10% FBS, 2 mmol/L L-glutamine, 100 IU/mL penicillin,100 μg/mL streptomycin, and 10 ng/mL recombinant human b-FGF. The cells were randomly assigned to control, as well as 5, 10, and 20 ng/mL VEGF-treated groups. The pre-induction medium was discarded, and cells from each group were washed with PBS and transferred to neuronal induction media comprising DMEM/F12, 4% FBS, 2 mmol/L L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. The control group was not treated with VEGF, and the three VEGF-treated groups were respectively treated with 5,10, and 20 ng/mL VEGF. The medium was replaced every 3 days. A phase-contrast microscope (× 200) was used to observe BMSC differentiation daily.
Immunocytochemistry
At 3 and 10 days after induction, cells from each group were fixed in acetone and absolute methanol at a volume ratio of 1: 1 at room temperature for 10 minutes. After washing twice in 0.01 mol/L PBS, the cells were incubated at room temperature with 3% bovine serum albumin for 30 minutes, followed by rabbit anti-NSE (1: 50)overnight at 4 °C. After several wash steps with 0.01 mol/L PBS, cells were incubated with biotin-labeled goat anti-rabbit IgG (1: 1) at room temperature for 15 minutes. After washing three times with 0.01 mol/L PBS,cells were incubated with an avidin-biotin conjugate of horseradish peroxidase at room temperature for 15 minutes, followed by diaminobenzidine (DAB) coloration,and washing with tap water after nuclear hematoxylin staining. The total number of NSE-positive cells was quantified from 20 randomly selected visual fields (× 200)per coverslip using phase-contrast microscopy. The experiments were performed in triplicate, and cells from three coverslips per group were selected for each experiment.
Main outcome measures
Morphological changes in BMSCs prior to and following VEGF induction; expression of the surface antigens CD90 and CD45 in BMSCs at passage 3 prior to VEGF induction; NSE expression following VEGF induction.
Design, enforcement and evaluation
The experiment was designed by Li Yi, Qiaoyun Liu, Jing Ye, and Fangting Zhang, performed by Li Yi, Qiaoyun Liu,and Jinling Han, and evaluated by Qiaoyun Liu, Jing Ye,Fangting Zhang, Guanghui Cui, and Zhuqing Zhou.
Statistical analysis
Statistical analysis was performed using SPSS 13.0(SPSS, Chicago, IL, USA). Data were expressed as Mean ± SD. Differences between groups were statistically evaluated using one-way analysis of variance. Independent samples t-test was used for comparison between two groups. P < 0.05 was considered statistically significant.
RESULTS
Morphological characteristics of BMSCs (Figure 1)

Figure 1 Morphology of undifferentiated bone marrow stromal cells (BMSCs). (A) At 3 d in primary culture, many cells are attached to the surface; the majority are round(red arrow), and a few are triangular (black arrow, × 200);(B) at 7 d in primary culture, spindle-shaped cells form colonies (black arrow, × 100); (C) cells at passage 3:BMSCs are relatively homogeneous in morphology, the majority are spindle-shaped (black arrow), with a whirlpool pattern, and some are large and flat (red arrow, × 100).
After 72 hours in culture, some adherent BMSCs were observed with varying appearances; primarily round cells accompanied by triangle and spindle-shaped cell bodies with short processes (Figure 1A). At 7–10 days in primary culture, the majority of spindle-shaped cells formed colonies (Figure 1B), and were used for passage. After 2–3 passages, the BMSCs were relatively homogeneous in appearance, and the majority of cells were spindle-shaped and displayed a whirlpool pattern; some cells were large and flat (Figure 1C).
ldentification of BMSC surface markers
Flow cytometry demonstrated that BMSCs from the third passage were positive for the stem cell marker CD90(97.5%) and negative for the hematopoietic cell marker CD45 (Figure 2).
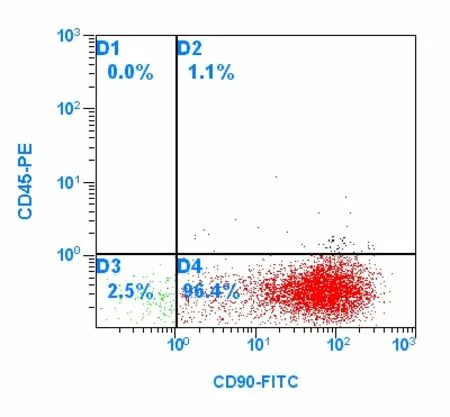
Figure 2 Detection of CD90 and CD45 expression in cultured bone marrow stromal cells by flow cytometry;96.4% of cells are CD90-positive and CD45-negative.
BMSC neuronal-like morphological changes following VEGF induction
Cells remained unchanged at 24 hours after pre-induction with bFGF. At 3 days following VEGF induction, the cells were shrunken, refractile, tapered, or round, and exhibited long, bipolar or multipolar, primary and secondary branches (Figure 3). Up to 10 days after induction, some cells in the control group displayed the same morphological changes.

Figure 3 Morphology of bone marrow stromal cells(arrow) at 10 d after induction. Cell bodies are refractile and tapered with primary and secondary branches (× 200).
lmmunocytochemical identification of neuronal differentiation
At 3 and 10 days after induction, flat BMSCs expressed very low levels of NSE protein, but the BMSCs with shrunken cell bodies and highly branched processes expressed higher levels of NSE (Figure 4). At 10 days after induction, the 10 ng/mL VEGF-treated group contained the greatest number of NSE-positive cells compared with the other groups (P < 0.05). The number of NSE-positive cells in the control group was least at 3 or 10 days post-induction (P < 0.05). The number of NSE-positive cells was significantly greater in all groups at 10 days compared with 3 days after induction (P <0.05; Table 1).
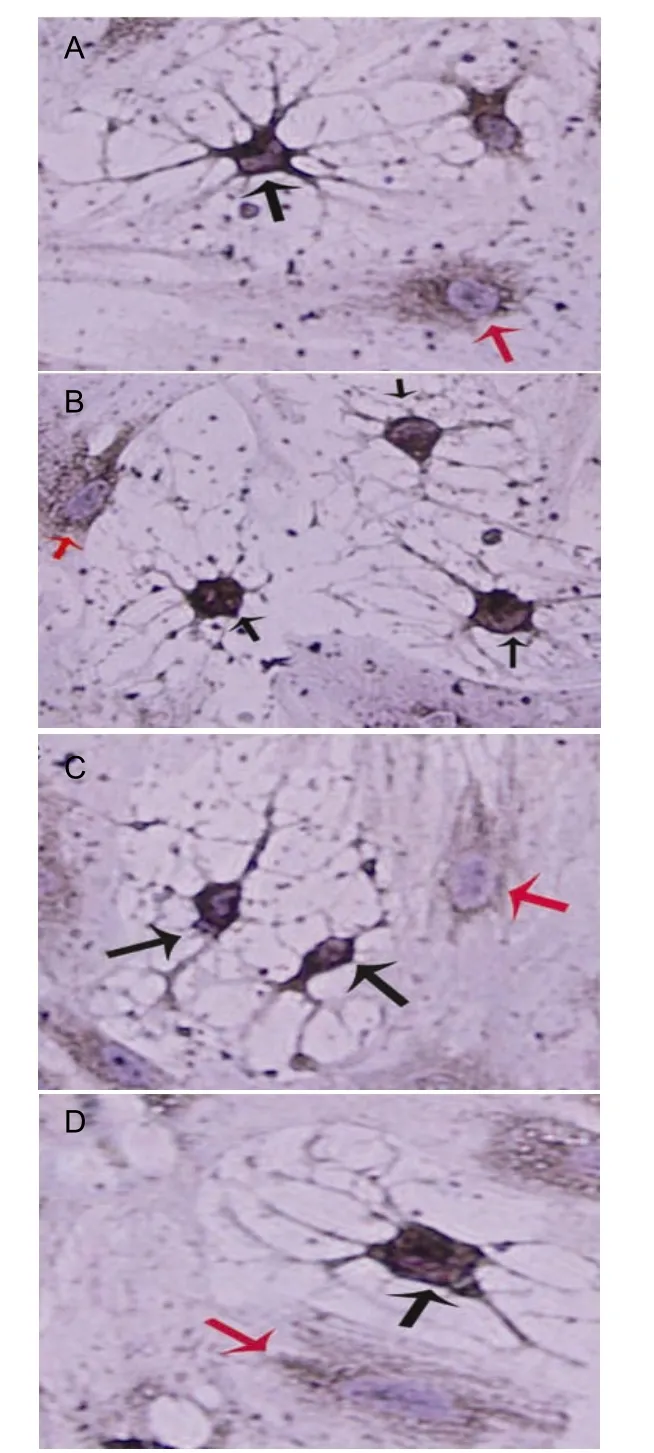
Figure 4 Neuron-specific enolase (NSE)-positive cells in all groups at 10 d after vascular endothelial growth factor(VEGF) induction. Images A-D represent 5, 10, and 20 ng/mL VEGF-treated, and control groups, respectively.Unresponsive bone marrow stromal cells (BMSCs) (red arrow) retain a flat appearance and express low levels of NSE. BMSC-derived neuronal-like cells (black arrows)express higher levels of NSE and exhibit condensed cell bodies with highly branched processes connected to adjacent cells. Nuclei are stained light blue by hematoxylin(× 400).
DlSCUSSlON
In the present study, BMSCs were isolated from rat bone marrow and successfully cultured in vitro. Growth properties and morphological characteristics of the BMSCs were similar to previous studies[7]. Flow cytometry results demonstrated that the majority of BMSCs at the third passage were CD90-positive and CD45-negative, which was in accordance with BMSC phenotype[16]and was similar to results from previous studies[17].
Table 1 Total number of neuron-specific enolase(NSE)-positive cells at 3 and 10 d after induction(±s, /20 fields, × 200)
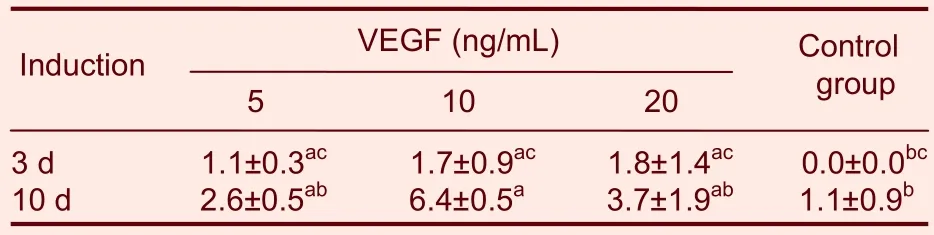
Table 1 Total number of neuron-specific enolase(NSE)-positive cells at 3 and 10 d after induction(±s, /20 fields, × 200)
aP < 0.05, vs. control group; bP < 0.05, vs. 10 ng/mL VEGF treated group; cP < 0.05, vs. VEGF group at 10 days; VEGF: vascular endothelial growth factor.
Control group 3 d 1.1±0.3ac 1.7±0.9ac 1.8±1.4ac 0.0±0.0bc 10 d 2.6±0.5ab 6.4±0.5a 3.7±1.9ab 1.1±0.9b VEGF (ng/mL)Induction 5 10 20
BMSCs from the third passage prior to induction were flat and spindle-shaped, but the cell bodies were shrunken and exhibited long processes following induction. Differentiated BMSCs, even from the control group, exhibited neuronal morphological characteristics accompanied by increased expression of the neuronal-specific marker NSE. These results suggested that rat BMSCs were capable of differentiating into neuronal-like cells, which was consistent with previous results[2].
Previous in vitro studies have demonstrated that VEGF stimulates axonal outgrowth and enhances cell survival and Schwann cell proliferation in the peripheral nervous system[18]. In the present study, the number of NSE-positive BMSCs was greater in the VEGF treated groups compared with the control group at 3 or 10 days post-induction, which suggested an important role of VEGF in BMSC neuronal differentiation.
Statistical analysis revealed the greatest number of NSE-positive cells in the 10 ng/mL VEGF-treated group at 10 days post-induction. Compared with 5 and 20 ng/mL, 10 ng/mL was the most appropriate VEGF concentration to induce in vitro neuronal differentiation of BMSCs. 5 ng/mL VEGF was possibly not sufficient to react with the specific receptors, and 20 ng/mL possibly interfered with receptor binding and signaling cascades related to differentiation of BMSCs. A concentration of 10 ng/mL VEGF was determined to be the most appropriate concentration. Moreover, the number of NSE-positive cells at 10 days was greater than at 3 days after induction in all groups, possibly due to increased stimulation of signaling cascades.
In addition, results from the three different VEGF concentrations demonstrated that only a few treated BMSCs NSE-positive, which suggests that only a few BMSCs have the potential to differentiate into neuronal-like cells.
This suggested that the obtained BMSCs were composed of tissue-specific progenitor cells, which could be capable of giving rise to cell specific expression in other tissues under appropriate conditions. Results from the present study demonstrated morphological heterogeneity of BMSCs, which was consistent with previous results[7].
In conclusion, VEGF induced BMSC differentiation into neuronal-like cells under certain in vitro conditions, and 10 ng/mL was the most appropriate VEGF concentration for induction. VEGF could be utilized to determine neuronal differentiation of BMSCs in vitro and repair following central neural damage and neural regeneration.
[1]Chen Y, Teng FY, Tang BL, et al. Coaxing bone marrow stromal mesenchymal stem cells towards neuronal differentiation:progress and uncertainties. Cell Mol Life Sci. 2006;63(4):1649-1657.
[2]Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364-370.
[3]Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature.2002;418(6893):41-49.
[4]Hermann A, Gastl R, Liebau S, et al. Ef fi cient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J.Cell Sci. 2004;117(19):4411-4422.
[5]Yang HJ, Xia YY, Lu SQ, et al. B-FGF indouced neuronal differentiation of mouse bone marrow stromal cells requires FGFR-1, MAPK/ERK and transcription factor or AP-1. J Biol Chem. 2008;283(9):5287-5295.
[6]Gendebien SW, Leprince P, Moonen G, et al. Regulation of neural markers nestin and GFAP expression by cultivated bone marrow stromal cells. J Cell Sci. 2003;116(16):3295-3302.
[7]Zhao L, Lin YD, Ma J, et al. Culture and neural differentiation of rat bone marrow mesenchymal stem cells in vitro. Cell Biol Int.2007;31(9):916-923.
[8]Dezawa M, Kanno H, Hoshino M, et al. Speci fi c induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113 (12):1701-1710.
[9]Ramos J S, Song S, Pelaez FC, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247-256.
[10]Suzukia H, Taguchia T, Tanakaa H, et al. Neurospheres induced from bone marrow stromal cells are multipotent for differentiation into neuron, astrocyte, and oligodendrocyte phenotypes. Biochem Biophys Res Commun. 2004;322(3):918-922.
[11]Conolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial growth and angiogenesis. J Clin Invest. 1989;84(5):1470-1478.
[12]Wang YM, Jin KL, Mao XO, et al. Greenberg VEGF overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85(4):740-747.
[13]Wang YQ, Guo X, Qiu MH, et al. VEGF overexpression enhances striatal neurogenesis in brain of adult rat after a transient middle cerebral artery occlusion. J Neurosci Res. 2007;85(1):73-82.
[14]Wang YQ, Cui HR, Yang SZ, et al. VEGF enhance cortical newborn neurons and their neurite development in adult rat brain after cerebral ischemia. Neurochem Int. 2009;55(7):629-636.
[15]The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[16]Gao YJ, Qian W, Wang BH, et al. Differentiation potential of Bone marrow stromal cells to enteric neurons in vitro. Chin J Dig Dis.2006;7(3):156-163.
[17]Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102(10):3483-3493.
[18]Zhang H, Hayashi T, Tsuru K, et al. Vascular endothelial growth factor promotes brain tissue regeneration with a novel biomaterial polydimethylsiloxane-tetraethoxysilane. Brain Res. 2007;1132(1):29-35.
