Hydrothermal Synthesisand Crystal Structure of a Supramolecular Compound[Ni(INAIP)(Him)(H2O)]◦H2O
2010-03-07CHENManshengDENGYifangCHENZhiinKUANGDaizhiYANGYingqun
CHEN Man-sheng,DENG Yi-fang,CHEN Zhi-m in,KUANG Dai-zhi,YANG Ying-qun
(1.Key Laboratory of FunctionalOrganometallic Materials,College of Hunan Province,Hengyang Hunan 421008,China;2.Department of Chemistry and MaterialsScience,Hengyang Normal University,Hengyang Hunan 421008,China)
Recently,the aim of contemporary crystalengineering is the development of new crystallinematerials with a variety of properties such as separation,magnetism,ion exchange,sensors,catalysis,gas storage and photoactivem aterials[1-8].How ever,the controlof formation of supramolecular complexes is still a fascinating challenge at present.Particularly,the organic ligands play crucial roles in determining the resulted polymeric structures,because the change in the type o f bridging units,the flexibility of the m olecular backbone,conformational preference,and symmetry of organic ligands can result in a remarkable class of materials bearing diverse architectures and functions.Our strategy in this app roach is using the organicmu liti-functional ligand 5-(isonicotinamido)isophthalic acid(H2 INA IP),which is sim ilar to the pyridine-3,5-dicarboxy late,only a few structures is known to date[9-10].In order to further investigate the influence of organic carboxylate ligandson the coordiantion architectures and related properties w ith imidazole ligands were carried out.Herein we report the synthesis,crystal structure of a new nickel coordination polymer,namely[Ni(INAIP)(H im)(H 2O)]◦H 2O(1).
1.1 Materials and instruments
The regents were used as commercial sources without further purification.Elemental analyses were performed on a Perkin-Elmer 240Celementalanalyzer.The ligand H2 INAIP was prepared as reported previously[10].The IR spectra were recorded on Bruker Vector22 FT-IR spectrophotometer using KBr discs.Thermogravimetric analyseswere performed on a simultaneous SDT 2960 thermal analyzer under nitrogen with a heating rate of10℃min-1.
1.2 Synthesis of the compound
Comp lex was synthesized by hydrothermal method in a 16 m L Teflon-lined autoclave by heating amixture of Ni(OAc)◦4H 2O(24.8 mg,0.1 mmol),H 2 INAIP(28.6mg,0.1 mmol),Him(8.0 mg,0.1mmol),8m LH2O and heated at140℃for 3 days.A fter cooling to room tem perature,green block crystals of the compound were collected in 32%yield.Anal.Calcd.for C17H15NiN4O7∶C 45.74;H 3.36;N 12.55;found:C 45.72;H 3.29;N 12.59%.IR(KBr pellet,cm-1):3 413(m),1 675(s),1 623(m),1 575(s),1 558(w),1 434(s),1 418(m),1 371(s),1 269(m),809(m),782(m),667(w),626(w),590(w).
1.3 X-ray crystallography
The X-ray diffraction measurement for the compond was performed on the Bruker Apex-ⅡCCD diffractometer with graphite-monochromated Mo-Kα radiation(λ=0.071 075 nm)at room temperature.The data were integrated by using the SAINT p rogram,which also did the intensity corrections for Lorentz and polarization effect.An empirical absorption correction was applied using the SADABS p rogram[11].Thestructureswere solved by directmethods using the p rogram SHELXS-97 and all the non-hydrogen atomswere refined anisotropically on F2by the full-matrix least-squares technique using the SHELXL-97 crystallographic software package[12-13].Crystal data and structure refinement parameters are listed in Table 1.The selected bond lengths and bond angles are given in Table 2.
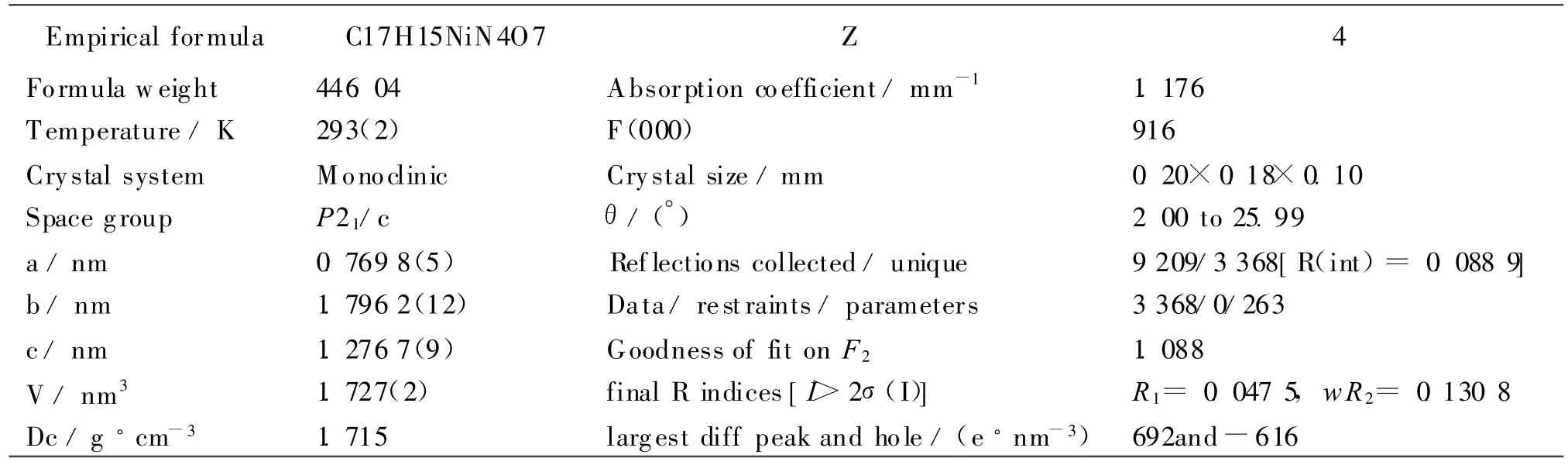
Tab le 1 Crystal Data and Structure Parameters for the Comp lex

Table2 Selected Bond Lengths(nm)and Bond Angle(°)
2.1 Structure description
The asymmetric unitof the compound consists of one Ni(Ⅱ)atom,one INAIP2-ligand,one imidazole ligand and one coordinated and one non-coordinated watermolecules.Each Ni(Ⅱ)atom is six-coordinated by two nitrogen(N1B and N3)atom from one INAIP2-and one imidzole ligand,one oxygen(O6)atom from the coordinated water molecule,and other three oxygen(O1,O2 and O4A)atoms from two different INAIP2-ligands,respectively,as shown in Fig.1.The coordination bond lengths and angles around the Ni(Ⅱ)are in the range of 0.209 8(3)-0.233 0(3)nm and 58.63(8)-175.81(9)°,respectively.Each INAIP2-ligand in turn coordinates w ith threemetal centers using its one pyridy land tw o carboxy late groups.The pyridylgroup coordinates to one Ni(Ⅱ)center and the two carboxylate groups link two Ni(Ⅱ)atomsw ith μ1-η1∶η1-chelating and μ1-η1∶η0-monodentate coordination modes,respectively.The coordinated watermolecu le acts as a terminal ligand to comp lete the octahedral coordination environment of the Ni(Ⅱ)atom.Therefore,the coordination interactions between the three-connecting INAIP2-ligand and six-coordinated Ni(Ⅱ)atom as described abovemake the compound a 2D layer,as illustrated in Fig.2.This is adistorted 2Dnetwork with(6,3)topology asillustrated in Fig.2,by treating the Ni(Ⅱ)atoms and the centers of the benzene rings of the INAIP2-ligand as three-connecting nodes,respectively.Furthermore,the N-H…O and O-H…O hyd rogen bonding interactions between the INAIP2-ligand and the free and coordinated watermoleculesextend the 2D layers into a 3D sup ramolecu lar net.
2.2 IR and TG Characterized
The infrared spectra of the title complexes have been recorded and some important assignments are shown above.No strong IR band from-COOH appeared at nearly 1 700 cm-1,indicating that the carboxy late ligands are protonated,the band at 3 413 cm-1,due to theν(O-H)absorptions of water molecules.These IR results are coincident with the crystallographic structural analyses.The results of thermogravimetric analyses(TGA)indicate that the compound lostits coordinated and non-coordinated watermolecules in the temperature range of 30~175 ℃(Fig.3).Theweight lossof8.01%is consistentwith calcu lated one of 8.07%.After the loss of all thewatermolecules,the supramolecular framework is stable up to 260℃,where the framework begins to decompose.

Fig.1 Molecular structure of the complex.All hydrogen atoms,freewatermoleculesareom itted for clarity

Fig.2 2D layer structure linked by INAIP ligands and Ni(Ⅱ)centerswith(6,3)topology
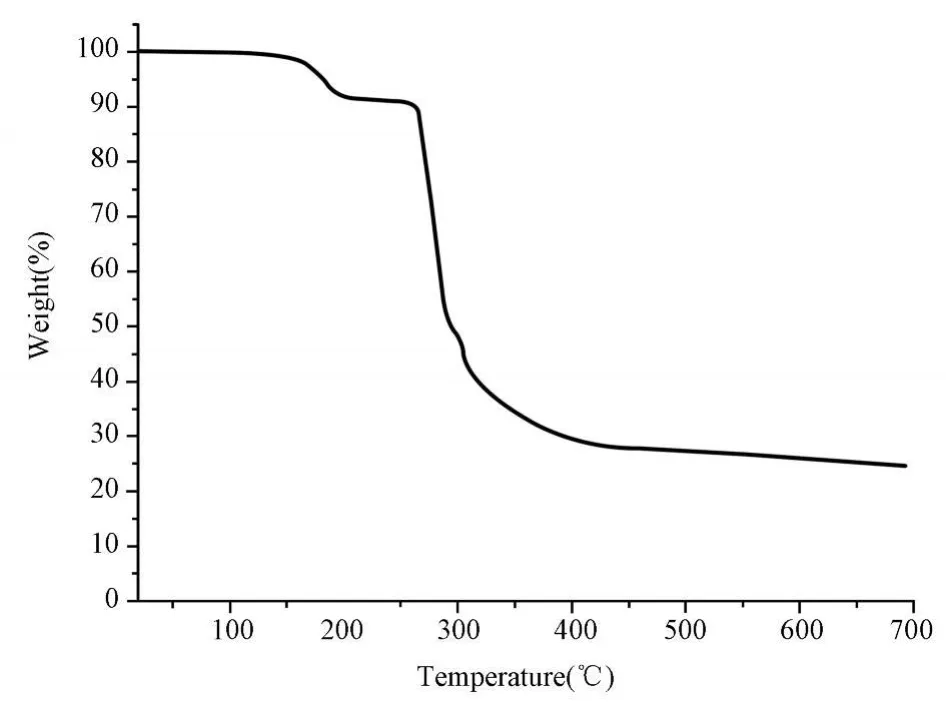
Fig.3 The TG curve of the compound

Table 3 Parameters of Hydrogen Bonds for the Complex 1(nm,°)
[1]Ockw ig,N.W.,Delgado-Fridrichs,O.,O'Keeffe,M,et al.Reticu lar chem istry:occurrence and taxonom y of nets and grammar for the design of framew orks[J].A cc.Chem.Res.,2005,38:176-182.
[2] Yamauchi Y,Youshizawa M,Fujita M.Engineering stacks of aromatic rings by the interpenetration of self-Assembled coordination cages[J].J.Am.Chem.Soc.,2008,130:5832-5833.
[3]Wu C D,H u A G,Zhang L,et al.A homochiral porous metal-organic framew ork for high ly enantioselec tive heterogeneous asymmetric catalysis[J].J.Am.Chem.Soc.,2005,127:8940-8941.
[4]Fang Q R,Zhu G S,Xue M,et al.Amine-tem p lated assembly o f metal-organic framew orks w ith attractive topologies[J].C ryst.Grow th Des.,2008,8:319-329.
[5]James S L.M etal-organic framew orks[J].Chem.Soc.Rev.,2003,32:276-288.
[6]Rosi N L,Kim J,Eddaoudi M,et al.Rod packings and metal-organic framew orks construc ted from rod-shaped secondary building units[J].J.Am.Chem.Soc.,2005,127:1504-1518.
[7]Eddaoudi M,K im J,Rosi N,et al.Systematic design of pore size and functionality in isoreticular MOFs and their app lication in methane storage[J].Science,2002,295:469-472.
[8]Yang S Y,Long L S,Jiang Y B,et al.An exceptionally stable metal-organic framew ork constructed from the Zn8(SiO4)core[J].Chem.Mater.,2002,14:3229-3231.
[9]Chen M S,Bai Z S,Su Z,etal.Three-dimensional fourfold interpenetrated(10,3)-b nickel(Ⅱ)framework with 5-(isonicotinamido)isophthalate[J].Inorg.Chem.Commun.,2009,12:530-533.
[10]Chen M S,Bai Z S,Okamura T-a,et al.M etal-organic framew orks w ith pyridy l-and carboxy late-containing ligands:syntheses,structures and properties[J].Cryst Engcomm,2010,12:1935-1944.
[11]Sheldrick G M.SADABS,Program for Bruker Area Detector Absorp tion Correction[CP].University of Göttingen:Göttingen,Germany,1997.
[12]Sheldrick G M.SHELXS-97,Program for Crystal Structure So lution[CP].University of Göttingen:Göttingen,Germany,1997.
[13]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement[CP].University of Göttingen:Göttingen,Germany,1997.
