D-和L-丙氨酸的低温磁相变——磁场变化下的比热和直流磁化率
2010-03-06王文清沈新春
王文清 沈新春 龚 䶮,2
(1北京大学化学与分子工程学院应用化学系,北京分子科学国家实验室,北京 100871; 2北京服装学院材料科学与工程学院,北京 100029)
D-and L-alanine are the basic molecules that make up all living organisms having a predetermined chirality[1-2].The inception of chirality from the elementary building blocks is one of the great mysteries of the origin of life.Alanines are orthorhombic crystals which exist as parallel chains of hydrogen bonded zwitterions(+NH3—C(R)H—CO-2)that form handed arrangement of N—H…O electric dipole moment.In amino acids,the N atom of the NH+3groups has the covanlency of four and is termed quadricovalent.There are two types of N—H bonds exhibiting different behaviors for hydrogen bonds.The structure notations used for these two types of hydrogen bonds are N—H…O and N+H…O which occur in amino acids.It is worthy noting that N+H…O bonds are usually stronger than N—H…O bonds[3-4].In recent paper[5],we have found that the spin flip-flop transition of N+H in D-and L-alanine occurred around 270 K, which was associated with NH+3group orientation plus N+H…O bond translation under the external magnetic field of 1 T(=10 kOe).In the present study we search for the lattice phonon and magnetic fieldeffectonthe heatcapacity from2 to 20 Kand expect to find the intrinsic asymmetries in D-and L-alanine crystals atcryogenic temperature.
1 Experimental
1.1 Materials
The polycrystalline powders of D-and L-alanine(Sigma Chemical Co.,99%)were recrystallized from sterilization aqueous solution at 277 K by slow evaporation for 2-3 weeks,producing well formed crystal elongated along the c axis,washed with absolute alcohol,evacuated and kept in a desiccator[6-7]. Crowell et al.[8]pointed out evaporation time of less than one week increased the inhomogeneous contribution to the phonon line shapes.However,no furtherimprovement in crystal quality was observed formore than three recrystallizations.Both D-and L-alanine crystals were determined by X-ray diffraction crystallography on a Rigaku RAXIS-RAPID imaging plate diffractometer with graphite monochromated Mo-Kαradiation(λ= 0.071069 nm)at 300,270,and 250 K[9].Neutron diffraction was performed at 295 and 60 K forD-,L-alanine and 300,270,and 250 K forD-alanine[10-11].The enantiomeric purity was identified by gas chromatography-mass spectrometry(GC-MS)of their N-trifluoroacetyl(TFA)isopropyl esters on Chirasil-Val capillary columns[12].Electrospray ionization-mass spectrometry(EI-MS, Bruker Daltonics,Billerica,USA)detected the organic impurities less than 2 fmol[13].FT-IR spectrometer(Nicolet iN 10 MX) with detection limits of 10 μg was used to preclude the possible H2Ocontentinmeasuring crystals.
1.2 Magnetic-field dependence of specific heat measurement
Alanine crystals were characterized by faces belonging to the families(020),(010),and(120),with the(120)faces always being the largest.The(020)faces were usually the smallest,but they appearedto be mostsuitable interms of optical quality.The crystal face and c axis of D-and L-alanine were ascertained by Laue method,XRD,and neutron diffraction.The magnetic-field dependence of specific heat of D-and L-alanine crystals were measured with a Quantum Design Physical Properties MeasurementSystem(PPMS-14)using the heat pulse-relaxation method. Both crystals were dried at room temperature for three days at 1.3×10-2Pa,in a system trapped with liquid nitrogen.Single crystals of D-alanine(16.13 mg)and L-alanine(16.31 mg)with the size of 3 mm×4 mm×0.5 mm were stuck by N-grease to the holder.Afterloading the systemthey were subjected to pumping at 1.3×10-3Pa for 3 h.High-precision specific heat measurements were performedoncrystals withthe same warming rate(1 K·min-1)from2 to 20 Kundermagnetic fields(H)with H//c axis in0,1,3 upto 5 T.
1.3 DC magnetic susceptibility measurement
DC magnetic moments were measured with a Quantum Design MPMS XL-5 SQUID system from 2 to 330 K.Due to the DC magnetic anisotropy,the principle susceptibility is not equal χa≠χb≠χc.The direction of magnetic axes is coincident with the alanine crystal axes a,b,and c.The strongest hydrogen bond N+H…O runs along the c axis.A pairof large D-/L-alanine crystals(390.89/338.50 mg)was selected and their sizes were reduced by grinding to fit the holder.The crystals were placed in the holder in such a way that the c axis was exactly parallel to the direction of the magnetic field.Based on the calculation of the orientational potential energy,an external magnetic field strength(H=1 T)was appliedparallel to the directionof N+H…O translation and then the magnetic moments(emu)were measuredby scanning three times.
2 Results and discussion
2.1 Enthalpy and entropy changes of D-and L-alanine between 2-20 K
The heat capacities of D-,L-alanine crystal are plotted as Cpvs T and Cpvs lnT(Fig.1)in the measured temperature range.At constantpressure,itis givenby the following equation:

Magnetic field dependence of heat capacity at 2-20 K forD-and L-alanine are listed in Tables 1,2.Based on the data in Table 1,magnetic-field dependence of the transition enthalpy and entropy changes were calculated by the usual method of graphical integration between 2 and 20 K and were listed in Table 3.The transition enthalpy ΔH and entropy ΔS of D-alanine are a little higher than that of L-alanine.It seems that the ground state of L-alanine is lower in energy than that of D-alanine,which illustrates an anticipated trend towards stabilization of the energetically lower enantiomer at interstellar temperature around2.7 K.
2.2 Boson peak of D-and L-alanine

Fig.1 Low temperature Cpversus T and Cpversus lnT of D-and L-alanine at zero-field

Table 1 Magnetic-field dependence of heat capacity at 2-20 K for D-alanine(16.13 mg)
The Boson peak resulted from the lattice vibrations,which forms on the basis of Debye theories.Rakvin et al.[14]have found the Boson peak of polycrystalline L-alanine in the low temperature region (1.8-20 K).In this experiment,the presence of the Boson peak was clearly shown forboth crystal lattices of D-and L-alanine single crystals as they progressed through theirrespective maxima in Cp/T3versus T in Fig.2.The positions of the maxima correlate with a hump in Cp/T3versus T plots.By fitting the curve of Cp/T3versus T,the temperature of Boson peak is 9.44 K for D-alanine and 10.86 K for L-alanine in zero-field with an uncertainty of 0.01 K (Table 4).At cryogenic temperature,the most of the intramolecular motions are frozen out and the residual dynamics should be related mainly to intermolecular motions in D-and L-alanine.The Boson peaks are intrinsic properties of the alanine lattice and the modes display a thermally activated dynamic orientational disorder of hydrogen-bonded network.We found that the polycrystalline L-alanine data by Rakvin et al.[14]showed the effect of different cooling rates which is evidence for disordered behavior.It helps explaining the discrepancy betweenthe Cpdatafrompolycrystalline powder andsingle crystal of alanine.
2.3 Debye temperature of D-and L-alanine
The Debye temperature ΘDis an important physical parameter of crystals,which defines a division line between quantum-mechanical and classical behavior of phonons[15].Heat capacity measurement at very low temperatures is a classical method for determining ΘD[16].At low temperature(below 10 K),the Debye model predicts[17],CV=aT3=(12/5)π4R(T/ΘD)3,where CVis heatcapacity at constant volume,R is gas constant.The Debye temperature follows the following relationship underconstant pressure, ΘD=12.480×(T/(Cp)1/3).
However,D-and L-alanine crystals did not obeyed Debye model absolutely at temperature range 2-20 K.By using curvefitequation from Cp/T3versus T plot(Fig.3),the Debye temperatures were calculated for the molecular crystals and the resultsare listed in Table 5.ΘDare 151.5 and 152.7 K for D-alanine andL-alanine inzero-field,respectively.

Table 2 Magnetic-field dependence of heat capacity at 2-20 K for L-alanine(16.31 mg)
It is shown that the magnetic contribution to the lattice heat capacity associated with the dependence of the Debye temperature on the magnetization.Since the interconnection of the Debye temperature with the magnetization has not yet been satisfactorily solved,this may be associated with the problem of separating the magnetic and electronic contribution in the low temperature region[18].
2.4 Magnetic contribution to the lattice heat capacity of D-and L-alanine——Schottky anomaly
The important signature of a zero-field splitting is the broad maximumin the specific heatwhich is called a Schottky anomaly[19].The magnetic contribution is superimposed on the lattice heatcapacity.The highertemperature tail of the Schottky specific heat varies as T-2.These results hold not only for a two-level systemathighertemperatures butforasystemwithnuclearspinelectronspinhyperfine interactions atvery low temperature.Theheat capacity data of D-and L-alanine lattice follow the T3law inthe measuredregion.The field splitting from2-12 Kprovides the magnetic contribution,which falls on top of the T3lattice term(Fig.2)[20].The total heat capacity in a situation should obey the relationship:Cp=aT3+b/T2(Table 6).Extrapolation to lower temperature allows an empirical evaluation of the lattice phonon contribution with aT3[21-22].The constants a and b can be calculated as:a=5.54×10-4,5.37×10-4J·mol-1·K-4,b=11.96, 11.65 J·K·mol-1for D-and L-alanine,respectively.All the magnetic contributions canbe includedinthe parameterb.
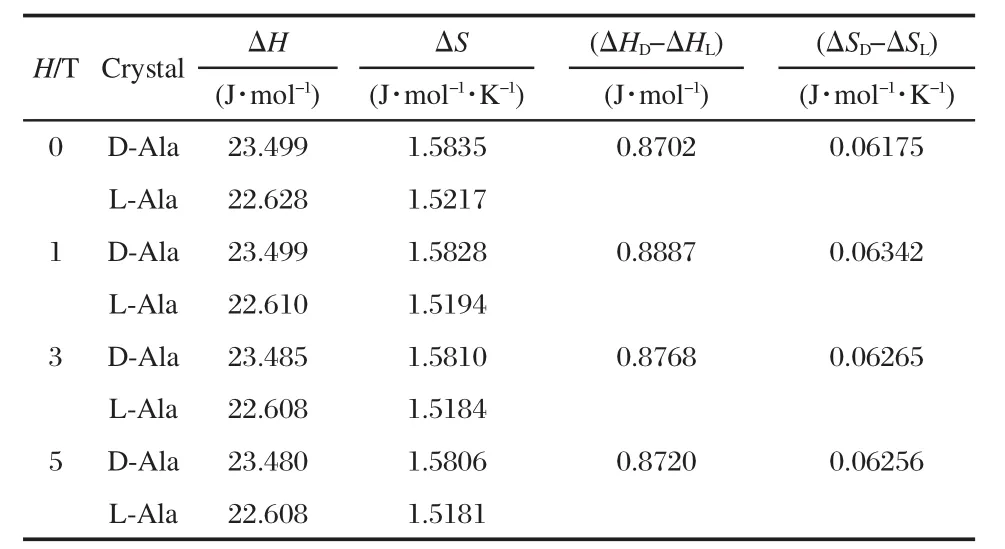
Table 3 Magnetic field dependence of ΔH and ΔS in D-and L-alanine crystals between 2 and 20 K

Fig.2 Temperature dependence of the heat capacities Cp/T3 of D-/L-alanine crystals as a function of external magnetic field with H//c-axis

Table 4 Temperatures of Boson peak of D-and L-alanine

Table 5 Debye temperature of D-and L-alanine

Table 6 Data of the equation Cp=aT3+b/T2for D-and L-alanine
We notice thatthe fourcurves of D-and L-alanine(H=0,1,3, 5 T)go togetheratthe temperature range 12-20 KinFig.2.Usually the phonon contribution in heat capacity does not vary with the magnetic field,therefore the heat capacity differences between these curves might come from spin contributions.The magnetic field dependence of the heat capacity was only observedbelow 12 Kwhichmeans thatthe spin systemwith nuclear spin-electron spin hyperfine interactions give contribution to heatcapacity of D-andL-alanine atvery low temperature.
2.5 Mechanism of magnetic transitions of D-and L-alanine around 12 K
The classical physicist has not considered the zwitterionic form in D-and L-alanine crystals.Compton et al.[23]depicted amino acidstructure thatbiologically consists of an amino group NH2,with a nitrogen atom N bonded to two hydrogen atoms, and a carboxylic acid group COOH,with a carbon atom bonded——actually double bonded——to one oxygen atom O and a hydroxyl groupOH,and to a central CHR group,where R varies instructure.However,Barron[24]depictedD-andL-alanine structures which existed in two distinguishable mirror images with zwitterionic forms as showninFig.4.

Fig.3 (Cp)1/3versus T of D-and L-alanine
In the case of zwitterionic form of amino acids,strong hydrogen bonds are formed betweengroups andgroups. The negative charge remains mainly localized on the oxygen atoms while the nitrogen and the nearby α-carbon atoms maintain a partial positive charge.Freedman et al.[25]proposed a vibrationally generated electronic ring current model for the case of L-alanine crystal.We supplement the D-alanine crystal model[9]. The Cα-H stretch generates an oscillating electronic current in a molecularring,adjacentto the methine bond,which is closed by hydrogen bond ring.This oscillating ring current gives rise to a large magnetic dipole moment,m,with a component in the direction of electric dipole moment μ.The rotational strength R=Im(μ·m),is positive in L-alanine and negative in D-alanine. The main difference between alanine enantiomers lies in the fact that they produce opposite optical rotation due to the opposite signof Im(μ·m)(Fig.5).

Fig.4 Two mirror-image enantiomers of alanine
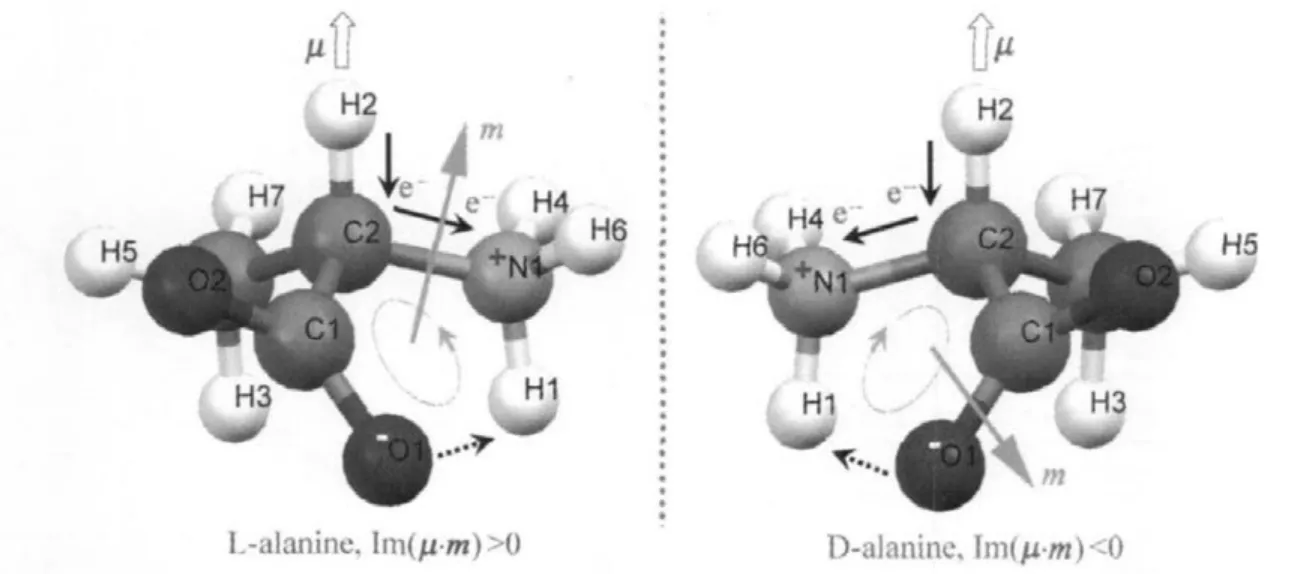
Fig.5 Freedman model:vibrationally generated ring currents in alanine enantiomers[9,25]
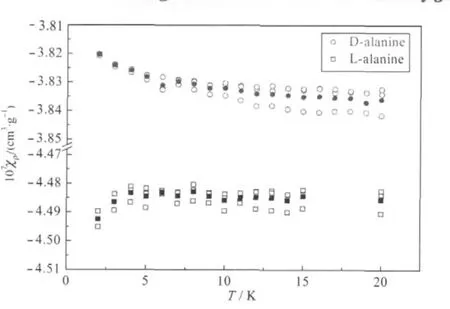
Fig.6 DC magnetic susceptibility of D-and L-alanine with H=1 T in the warming process from 2 to 20 Kopen symbol:three times scanning,solid symbol:mean value
Based on the new definition of“True and False Chirality”by Barron[26],Compton et al.[23]illustrated a rotating ammonia molecules like a spinning cone translating along the axis of the cone and representing a truly chiral system.However,Compton wondered that ammonia is rapidly converting from one to the other, requires that the two states would have definite chirality but not definite parity.
According to the studies of the internal rotation in solid αglycine[27],whichshowedinternalmotionoftheNH+3groupchanging from a rigid structure at temperatures below 100 K to a rotation at temperature above 200 K.Internal rotation barriers were (14.4±0.8)kJ·mol-1forthe NH+3and(255±12)kJ·mol-1forthe COO-group.Proton and deuteron magnetic resonance results quantitatively confirm the zwitterion nature of α-glycine[28-29].The linewidth transitions indicate the presence of two types of NH3groups in achiral α-glycine with different potential barriers,one reorienting fast and the otherslow.McDermott et al.[30]reported that the energy barriers for amine rotation are(24±2)kJ·mol-1forα-glycine and(40±2)kJ·mol-1forL-alanine.
Withthe view of understanding this dilemma,amagnetic field H=1 T was applied parallel to the direction of the strongest hydrogen bonds of N+H…Otranslation(along c axis)and the magnetic moments(emu),magnetic susceptibilities(χρ,cm3·g-1)was measured by scanning three times(Fig.6).The magnetic susceptibilities data between D-and L-alanine illustrate an opposite trend atvery low temperature(2-12 K).Therefore,the DC magnetic susceptibility results of D-and L-alanine showed Boson peakof nuclearspin-electronspinhyperfine interactionbelow 10 K.
We have found the Boson peak around 12 K on the phase transition of D-and L-alanine by magnetic-field dependence of heat capacity with PPMS-14.DC-magnetic susceptibility data with MPMS-XL5 demonstrated the nuclear spin-electron spin hyperfine interaction at very low temperature(2-12 K).According to the heat capacity and DC-magnetic susceptibility measurements[3],the magnetic chirality transitions of D-and L-alanine were observed on the pre-aligned molecules in a single crystal.
3 Conclusions
In summary,this study notonly provides an efficientand novel probe to monitorthe space and time odd effects on the phase transitions of alanine enantiomers,but also supports logically and correlates itself systematically with different electron spin and nuclearspin properties of enantiomers nearthe phase transition around 2-12 K.Intensive study about the temperature effects on the origins of parity violating energy difference of electron spin and nucleus spin properties between enantiomers such as D-and L-valine,achiral α-glycine(containing two types of NH3group),may leadto clues forthe betterunderstanding of the underlying physical law of space and time violations in dynamic molecularprocesses.
1 Fisher,G.H.Amino Acids,2007,32:1
2 Morikawa,A.;Hamase,K.;Inoue,T.;Konno,R.;Zaitsu,K.Amino Acids,2007,32:13
3 Murli,C.;Thomas,S.;Venkateswaran,S.;Sharma,S.M.Physica B,2005,364:233
4 Fuller,W.J.Phys.Chem.,1959,63:1705
5 Wang,W.Q.;Shen,X.C.;Gong,Y.Acta Phys.-Chim.Sin.,2008, 24:743 [王文清,沈新春,龚 䶮.物理化学学报,2008,24: 743]
6 Simpson,H.J.;Marsh,R.E.Acta Cryst.,1966,20:550
7 Destro,R.;Marsh,R.E.J.Phys.Chem.,1988,92:966
8 Crowell,R.A.;Chronister,E.L.J.Phys.Rev.B,1993,48:172
9 Wang,W.Q.;Min,W.;Bai,F.;Sun,L.;Yi,F.;Wang,Z.M.;Yan, C.H.;Ni,Y.M.;Zhao,Z.X.Tetrahedron-Asymmetry,2002,13: 2427
10 (a)Wang,W.Q.;Liu,Y.N.;Gong,Y.Acta Phys.-Chim.Sin., 2004,20:1345 [王文清,刘轶男,龚 䶮.物理化学学报,2004, 20:1345] (b)Wang,W.Q.;Gong,Y.;Yao,N.Acta Phys.-Chim.Sin.,2005, 21:774 [王文清,龚 䶮,姚 楠.物理化学学报,2005,21: 774]
11 Wilson,C.C.;Myles,D.;Ghosh,M.;Johnson,L.N.;Wang,W.Q. New J.Chem.,2005,29:1318
12 Cronin,J.R.;Pizzarello,S.Science,1997,275:951
13 Gledhill,M.Analyst,2001,126:1359
14 Rakvin,B.;Maltar-Strmecki,N.;Ramsey,C.M.;Dalal,N.S. J.Chem.Phys.,2004,120:6665
15 Jasiukiewicz,C.;Karpus,V.Solid State Commun.,2003,128:167
16 Pantea,C.;Stroe,I.;Ledbetter,H.;Betts,J.B.;Zhao,Y.;Daemen, L.L.;Cynn,H.;Migliori,A.J.Phys.Chem.Solids,2008,69:211
17 Drebushchak,V.A.;Kovalevskaya,Y.A.;Paukov,I.E.; Boldyreva,E.V.J.Therm.Anal.Calorim.,2006,85:485
18 Bodryakov,V.Y.;Povzner,A.A.;Zelyukova,O.G.Physics of the Solid State,1999,41:1138
19 Carlin,R.L.Magnetochemistry.Berlin,Heidelberg,Germany: Springer-Verlag,1986:41-42
20 Dhar,S.K.;Gschneidner,K.A.;Bredl Jr.,C.D.;Steglich,F.Phys. Rev.B,1989,39:2439
21 O′connor,C.J.;Bhatia,S.N.;Carlin,R.L.Physica B,1978,95: 23
22 Zheng,P.;Luo,J.L.;Wu,D.;Su,S.K.;Liu,G.T.;Ma,Y.C.; Chen,Z.J.Chin.Phys.Lett.,2008,25:3406
23 Compton,R.N.;Pagni,R.M.Advances in Atomic,Molecular and Optical Physics.,2002,48:219
24 Barron,L.D.Space Sci Rev.,2008,135:187
25 Freedman,T.B.;Balukjian,G.A.;Nafie,L.A.J.Am.Chem.Soc., 1985,107:6213
26 Barron,L.D.Chem.Soc.Rev.,1986,15:189
27 Li,R.C.J.;Berman,N.S.J.Phys.Chem.,1970,74:1643
28 Saraswati,V.;Vijayaraghavan,R.J.Phys.Soc.Jap.,1967,23: 590
29 Reitboeck,H.Biophysik,1967,4:15
30 Gu,Z.T.;Ebisawa,K.;McDermott,A.Solid State Nucl.Magn. Reson.,1996,7:161
