肿瘤靶向腺相关病毒携带干扰素β和TRAIL对A549肺癌移植瘤的增强治疗效应
2010-02-10王毅刚何凌峰何国清孔彦平刘旭平蔡海波刘新垣谭文松
王毅刚,何凌峰,何国清,孔彦平,刘旭平,蔡海波,刘新垣,谭文松
1 华东理工大学 生物反应器工程国家重点实验室,上海 200237
2 浙江理工大学生命科学学院 新元医学与生物技术研究所,杭州 310018
3 中国科学院上海生命科学院 生物化学与细胞生物学研究所 分子细胞生物学实验室,上海 200031
Introduction
Adeno-associated virus (AAV) is a member of the Parvovirdae family, which presently has been deemed to be one of the most promising gene therapy vectors. The characteristic of AAV mainly includes few or null immune response, non-pathogenicity, wide tissue or cell tropisms, long-term and stable transgene expression etc[1]. Currently, among all used AAV serotype, AAV2 was the most usual gene delivery vector. However, the wide host range made AAV2 short of tissue or cell specificity in cancer gene therapy. To improve the targeting cancer therapeutic effect of AAV, there are mainly two approaches to be attempted, including transcriptional targeting strategy and transduction targeting strategy, which is the use of tumor-specific promoters and the tropism modification of AAV capsids, respectively[2]. Several data has showed that the application of human telomerase reverse transcriptase (hTERT) could effectively improve the therapeutic effect in targeting cancer therapy[3-4].
Human interferon β (IFN-β) is type I IFNs and one of the cytokines earliest for clinical cancer therapy. Presently, IFN-β has been widely used as a clinical anticancer agent on malignant glioma, melanoma, colorectal cancer, renal cell carcinoma, etc[5-6]. The pleiotropic antitumor mechanisms are involved in the immunmodulatory activity to tumor through upregulation of MHC class I expression, activation of Natural Killer cell and cytotoxic T lymphocyte[7-9], the inducement of tumor cell apoptosis and inhibition of tumor angiogenesis et al[10-11]. Many studies showed IFN-β has a significant antitumor effect both in vitro and in vivo via gene therapy methods or recombinant protein[11-12]. TNF-related apoptosis-inducing ligand (TRAIL) is one member of tumor necrosis factor (TNF) superfamily and currently is under development as a potential therapeutic agent because it can specifically induce apoptosis of various cancer cells through two pathways including dependent on or undependent on mitochondria, while no significant toxic side effects to the vast majority of normal cells[13]. The tumor targeting virus AAV-hTERT-IFN-β or AAV-hTERT-TRAIL previously constructed by us, which using the hTERT promoter to control AAV-mediated IFN-β or TRAIL gene expression, resulted in cancer-specific cell killing effect and inhibited the xenograft growth of lung cancer, colon cancer and hepatocellular carcinoma in nude mice model[14-15]. However, the single gene therapy has no significant antitumor effect[16]. Some studies reported that the two genes of different mechanisms can play a synergistic or complementary antitumor effect[17]. In the treatment of melanoma, the combined treatment of IFN-β and TRAIL gene led to more apoptosis and improved inhibitory effect of tumor growth than the alone[18], which suggested that they have a synergistic mechanism of action. Up to date, there is no report available that combines tumor targeting AAV-mediated dual gene (IFN-β and TRAIL) therapy of cancer.
This study is based on the construction of AAV-hTERT-IFN-β and AAV-hTERT-TRAIL, and explored their inhibitory effect on lung cancer cell growth in vitro and in vivo by combined IFN-β and TRAIL. The results showed that the combinational antitumor effect by AAV-hTERT-IFN-β and AAV-hTERT-TRAIL is more evident than any the alone in both tumor cell lines and an animal model of lung cancer xenografts, and it lay a foundation for exploring the antitumor activity and the molecular mechanisms of IFN-β and TRAIL dual gene.
1 Materials and methods
1.1 Cell lines and culture
HEK293 cell line (human embryonic kidney containing the E1 region of Ad5) was obtained from Microbix Biosystems Inc. (Toronto, Canada). Human lung fibroblast cell line MRC5, human lung cancer cell line A549, human hepatocellular carcinoma cell line BEL7404 and human cervical cancer cell line HeLa were purchased from Shanghai Cell Collection (Shanghai). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-Diphenyl tetrazolium bromide) and Hoechst33258 apoptosis detection kit were purchased from Sigma company (St. Louis, MO, USA) and Beyotime (Nantong), respectively. The MRC5 and HEK293 cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco BRL, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco BRL). The A549, BEL7404 and HeLa cell lines were grown in DMEM supplemented with 5% heat-inactivated FBS at 37°C in a humidified atmosphere with 5% CO2in air.
1.2 Vector construction and rAAV production
AAV vector plasmids were constructed with hTERT promoter (from Dr. Fang, University of Texas M.D.) to control transgene expression instead of CMV promoter to form pAAV-hTERT-Genes. The enhanced green fluorescent protein (EGFP) gene, IFN-β gene, and TRAIL gene were inserted into the vector plasmid pAAV-hTERT-Genes for respective construction of pAAV-hTERT-EGFP, pAAV-hTERT-IFN-β and pAAV-hTERT-TRAIL, which were preserved in our labortary[15-16]. For AAV production, the packaging plasmid pDG were employed. All virus preparations were purified by CsCl gradients to ensure purity and titers of AAV virus vectors were determined by PCR according to previous studies[16]. The titration of AAV-hTERT-EGFP, AAV-hTERT-IFN-β and AAV-hTERT-TRAIL is 5×1012v.g/mL (virus genomes/mL), 2×1012v.g/mL and 2×1012v.g/mL, respectively.
1.3 Transduction assay of reporter gene in vitro
Cells were plated at a density of 105cells in 6-well plates and infected with AAV-hTERT-EGFP at a multiplicity of infection (MOI) of 105v.g/cell. After infection for 24 h, 48 h, 72 h, respectively, EGFP expression was detected by fluorescence microscope with a digital camera apparatus DP70 (Olympus, Japan).
1.4 Cell viability assay
Three tumor cell lines A549, BEL7404, HeLa and normal cell line MRC5 were seeded on 96-well plates at a density of 1×104per well. When cells adhered, they were infected with AAV-hTERT-EGFP, AAV-hTERT-IFN-β, AAV-hTERT-TRAIL and the combination with AAV-hTERT-IFN-β and AAV-hTERT-TRAIL. MTT assay was used to measure the cell viability. Briefly, 20 µL MTT (5 mg/mL in PBS) was added to each well 72 h after infection. After 4 h at 37°C, media and MTT were lightly removed and 150 µL DMSO was added. Absorbance was read at 595nm with an ELISA reader (Molecular Devices, Sunnyvale, CA).
1.5 Detection of IFN-β expression by enzymelinked immunosorbent assay (ELISA)
The expression of secreted IFN-β in media was measured using human IFN-β ELISA Kit (Biomedical Laboratories, Piscataway, NJ). Tumor cells and normal cells were infected with AAV-hTERT-IFN-β at a MOI of 103, 104, and 105v.g/cell. Diluted standards and supernatants containing IFN-β protein were added at 100 µL/well in 96-well plates coated with anti hIFN-β antibody. All procedures complied with the manufacture’s instruction and the optical density (OD) of the plates was measured at a wavelength of 450 nm.
1.6 Flow cytometry analysis
Cells were seeded on 6-well plates at a density of 5×105cells/well. Virus infection was performed with a MOI of 105v.g/cell. Cells were harvested 72 h after infection and washed once with complete medium. Aliquots of cells were resuspended in 500 µL binding buffer and stained with fluorescein isothiocyanate (FITC)-labeled annexin V (BioVision, Palo Alto, CA) according to the manufacturer’s instructions. A fluorescence-activated cell-sorting (FACS; Becton Dickinson) assay was performed immediately after staining.
1.7 Apoptotic cell staining
Cells seeded in 24-well plates were treated with various groups. After 72 h of treatment, the cells were incubated with Hoechst 33258 for 30 min, washed with PBS twice, and observed and took photos under a fluorescence microscope.
1.8 Animal experiments
All animals used in the experiments were maintained at institutional facilities in accordance with the regulations and standards of U.S. Department of Agriculture and National Institutes of Health. Female BALB/c nude mice (4−5 weeks of age), obtained from Animal Research Committee of the Institute of Biochemistry and Cell Biology (Shanghai), were used in all of the experiments. Aliquots of A549 cells (5×106, suspended in 100 µL PBS) were subcutaneously inoculated into the lower right flank of the nude mice. When the tumors were 100−150 mm3in size after 2 weeks of tumor implantation, mice were randomized into five groups and were administrated with AAV-hTERT-EGFP, AAV-hTERT-IFN-β, AAV-hTERT-TRAIL and AAV-hTERT-IFN-β plus AAV-hTERT-TRAIL at a dose of 2×1012v.g/kg respectively or PBS via intratumoral injection every second day for three times. Tumor growth was monitored using calipers every week. Tumor volume (V) was calculated by using the formula: tumor volume V (mm3) = 1/2×Lenth (mm) ×Width (mm)2. At the end of the experiment, tumor tissues were harvested for additional analyses as described below. Differences in tumor growth were tested for statistical significance.
1.9 HE staining and immunohistochemistry assay
Tumor tissues were fixed in 4% formaldehyde, dehydrated with gradient ethanol, and embedded in paraffin. Tissue sections (5 µm) were then dewaxed and rehydrated according to a standard protocol. For Hematoxylin and Eosin (HE) analysis, sections were stained with hematoxylin and eosin. For Immunohistochemistry (IHC) assay, the sections were washed with PBS, treated with 3% H2O2at room temperature, blocked at 37°C for 20 min and then followed by incubation with human monoclonal anti-IFN-β antibody (diluted 1:200; Chemicon Inc. Single Oak Drive-Temecula) overnight. After incubation with rabbit anti-human secondary antibody, expression of intracellular IFN-β was detected with diaminobenzidine (DAB; Sigma) by enhancement with an avidin-biotin reaction ABC kit (Bio-Genex laboratories, CA). The slides were then counterstained with hematoxylin.
1.10 Statistical analysis
2 Results
2.1 Tumor-selective killing effect by different viruses controlled by hTERT promoter
The tumor targeting AAV-hTERT-gene system was constructed for this study. The human IFN-β gene and TRAIL gene were inserted into pAAV-hTERT-gene to form pAAV-hTERT-IFN-β and pAAV-hTERT-TRAIL, respectively. The construction of pAAV-hTERT-EGFP was used as control. The packaging and purification of recombinant AAV2 were generated as described previously[15-16], and the titer of rAAV were presented as virus genomes (v.g)/mL. First of all, in order to prove that rAAV-mediated gene expression driven by hTERT promoter has tumor targeting ability, we detected tumor-specific expression of IFN-β protein by ELISA analysis in cultured cell line. The results showed that the expression of IFN-β is very obvious in A549 cells after transduced with AAV-hTERT-IFN-β but seldom in normal MRC5 cells (Fig. 1). The secreted concentration of IFN-β protein was dose-dependent, and its expression in A549 cells infected by 105v.g/cell of AAV-hTERT-IFN-β was the maximum and reached 1866 pg/mL compared with 255 pg/mL in normal MRC5 cells. The specific EGFP expression was also detected in tumor cell lines. As shown in Fig. 2, green fluorescence can be observed in A549 cells but seldom in MRC5 cells after transduced with AAV-hTERT-EGFP, and was time-dependent. These results suggested that hTERT promoter which controlled gene expression mediated by rAAV had the potential tumor targeting ability.
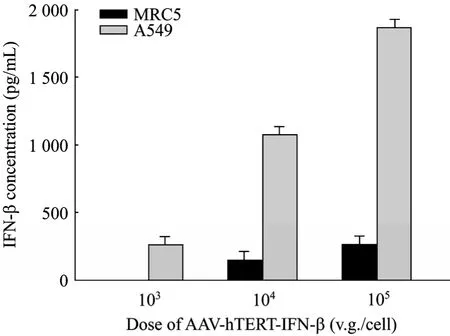
Fig. 1 Tumor-specific IFN-β expression in A549 lung cancer cells transduced by AAV-hTERT-IFN-β. The normal cell line MRC5 and tumor cell line A549 were infected with AAV-hTERT-IFN-β at a gradient of MOI 103, 104, and 105 v.g/cell, respectively. The expression of secreted IFN-β protein was detected by ELISA assay after 72 h. The results were represented as ±s. Data were summarized from three experiments and expressed as histogram to reflect the secreted IFN-β concentration after transduction of different dose AAVs.
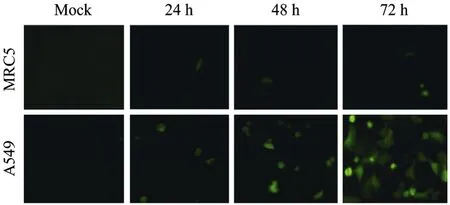
Fig. 2 Tumor-specific EGFP expression by AAV-hTERT-EGFP transduction. The normal cell line MRC5 and tumor cell line A549 were infected with AAV-hTERT-EGFP at a MOI of 105 v.g/cell. The green fluorescent cells were observed under fluorescence microscopy after 24 h, 48 h and 72 h, respectively.
Previous studies showed that either AAV-hTERTIFN-β or AAV-hTERT-TRAIL specifically suppressed tumor cell growth in vitro. Based on them, to further improve the ability for killing tumor cells, we tested the effect on tumor cell viability by combined use of AAV-hTERT-IFN-β and AAV-hTERT-TRAIL. Tumor cell lines (BEL7404, HeLa, and A549) and normal cell line (MRC5) were infected with various viruses at a dose of 104v.g/cell, and cell viability was analyzed by MTT assay. As shown in Fig. 3, the cell viability in tumor cell lines treated with a combination of AAV-hTERT-IFN-β and AAV-hTERT-TRAIL decreased to approximately 60%−80%, compared with AAV-hTERT-EGFP, AAV-hTERT-IFN-β, or AAV-hTERTTRAIL. About 79% of A549 cells and 69% of BEL7404 cells were killed by the combinational treatment after 72 h, but such a phenomenon could not be observed in other treatments. Among three tumor cell lines, A549 cell line is the most sensitive with only 21% survival cells. No significant changes were observed for normal MRC5 cells viability. Moreover, the cytotoxic effect of AAV-hTERT-EGFP was less apparent in the tested cell lines than other treatments, suggesting the virus itself is safe. These data indicated IFN-β and TRAIL armed with AAV controlled by hTERT promoter exhibited the additive tumor-specific killing effect and had the potential antitumor effect in animal experiments.
2.2 Induction of apoptosis by AAV-hTERT-IFN-β and/or AAV-hTERT-TRAIL
AAV-hTERT-IFN-β or AAV-hTERT-TRAIL could efficiently induce the apoptosis of tumor cells, respectively, as shown in previous study[15-16]. To evaluate the effect of apoptosis by the combination of AAV-hTERT-IFN-β and AAV-hTERT-TRAIL, we first observed the apoptotic morphological changes of tumor cells by Hoechst33258 staining after each treatment using a fluorescence microscope. More tumor cells treated by the combination of AAV-hTERT-IFN-β and AAV-hTERT-TRAIL showed obvious apoptosis features including chromatin condensation and nuclear fragmentation (arrows indicate the apoptotic cells), compared with other groups. However, normal MRC5 cells had no significant apoptotic morphological changes after treatment of different viruses (Fig. 4). Further, we performed flow cytometry analysis to detect the percentage of apoptotic cells by annexin V staining. The results were consistent with the morphological changes. The combined treatment of AAV-hTERT-IFN-β and AAV-hTERT-TRAIL led to a higher percentage of cell apoptosis in A549 cells and reached almost 50% compared with AAV-hTERT-IFN-β alone and AAV-hTERT-TRAIL alone (Fig. 5). Together these, our results confirmed that the inhibition of tumor cell growth mediated by dual tumor targeting virus AAV-hTERT-IFN-β plus AAV-hTERT-TRAIL was involved with the apoptotic process.
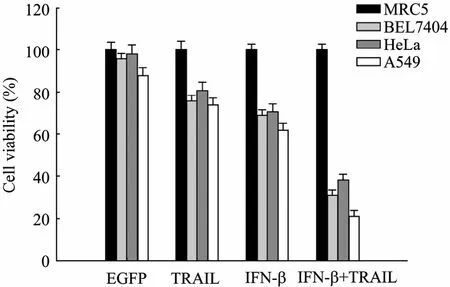
Fig. 3 Tumor cell viability after different AAVs transductions in vitro. The normal cell line MRC5, three tumor cell lines BEL7404, HeLa, and A549 seeded in 96-well plate were infected with AAVs at a MOI of 104 v.g/cell. Cells with PBS treatment were used as control. Cells were treated with MTT after 72 h. Experiments were repeated by 3 times and data expressed as histogram to reflect the cell viability after different AAV transductions. Transduced with AAV-hTERT-EGFP (EGFP), AAV-hTERT-TRAIL (TRAIL), AAV-hTERT-IFN-β (IFN-β), and the combination of AAV-hTERT-TRAIL and AAV-hTERT-IFN-β (IFN-β+TRAIL), respectively.
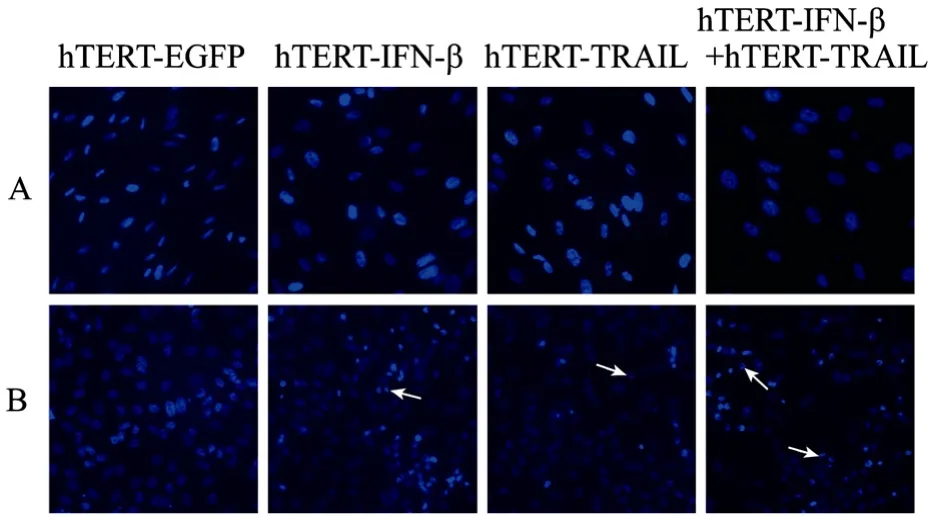
Fig. 4 Observation of apoptotic morphological changes by Hoechst33258 staining. MRC5 cells (A) and A549 Cells (B) were infected by AAV-hTERT-EGFP, AAV-hTERT-TRAIL, AAV-hTERT-IFN-β, and the combination of AAV-hTERTTRAIL with AAV-hTERT-IFN-β at a MOI of 104 v.g/cell for 48 h. Apoptotic change of tumor cells was observed by fluorescence microscopy, as arrows indicate.

Fig. 5 Percentage of apoptosis was measured by Annexin V staining. A549 cells were harvested after infection as the same with MTT analysis. Infected cells were stained with Annexin V-FITC and immediatedly followed by flow cytometry for apoptosis assay. The percentage of apoptotic cells was calculated with CellQuest software. Each value represents the mean of 3 wells.
2.3 Antitumor efficacy of AAV-hTERT-IFN-β and AAV-hTERT-TRAIL in vivo
Both IFN-β and TRAIL are recognized as potent antitumor agent for their pleiotropic antitumor mechanisms. IFN-β can induce apoptotic cell death in lymphoma cells or ovarian carcinoma cells through TRAIL, which imply that the combined treatment of IFN-β and TRAIL caused better therapeutic effect on tumor growth than the alone[18-20]. To determine whether AAV-hTERT-IFN-β plus AAV-hTERT-TRAIL can induce enhanced antitumor activity in vivo, we established subcutaneously transplanted mice model with A549 lung cancer cells. Tumors were administrated by different viruses or PBS, and tumor growth curves were plotted to compare the difference of their antitumor efficacy. The results indicated that there was a rapid decrease in mean tumor volume in receiving intratumoral injections of AAV-hTERTIFN-β, AAV-hTERT-TRAIL, and their combination compared with those receiving injections of PBS, AAV-hTERT-EGFP alone (Fig. 6A). Tumor growth was significantly inhibited after receiving intratumoral injection of AAV-hTERT-IFN-β plus AAV-hTERTTRAIL, and the inhibition was more profound than that of AAV-hTERT-IFN-β alone or AAV-hTERT-TRAIL alone. The tumor volume in the combinational group only was 52 mm3after 10 weeks of treatment than 287 mm3of AAV-hTERT-IFN-β and 294 mm3of AAV-hTERT-TRAIL. Moreover, intratumoral injection of AAV-hTERT-IFN-β, AAV-hTERT-TRAIL or their combination resulted in an improved survival rate compared with PBS, AAV-hTERT-EGFP groups (Fig. 6B). Only one mouse died 63 days after treatment with AAV-hTERT-IFN-β, whereas all established lung tumor xenografts were almost eliminated, and all nude mice survived until euthanasia on day 91 after receiving a combined injection of AAV-hTERT-IFN-β with AAV-hTERT-TRAIL. The results proved that their combination has significant enhanced antitumoral potential in vivo.

Fig. 6 Antitumor effect of different AAV injections on A549 tumor xenografts in vivo. Tumors were established by injecting A549 cells (5×105) subcutaneously into nude mice. (A) Tumor volume was observed for 13 weeks in mice with tumors after intratumoral injection with PBS, AAV-hTERT-EGFP, AAV-hTERT-TRAIL, AAV-hTERT-IFN-β, and their combinations, respectively. Each point represents the ±sof each group (n=8). Statistical significance: P<0.001 for the combinational treatment to AAV-hTERT-IFN-β; P<0.01 for the combinational treatment to AAV-hTERT-TRAIL. (B) Kaplan-Meier survival curves of animals after the same treatments as above. Pair-wise log-rank test was used to analyze survival rates in different groups.
2.4 Detection of tumor cell death and Immunohistochemistry of IFN-β in tumor tissues
To further verify the possibility that antitumor effect of AAV-hTERT-IFN-β was due to the IFN-β overexpression in tumor xenografts, the presence of human IFN-β was examined by IHC staining using anti-human IFN-β antibody. It is evident that there was a strong expression of IFN-β in all xenografts with the injection of AAV-hTERT-IFN-β and that the highest expression of IFN-β was observed in tumor sections that was received combined injections of AAV-hTERT-IFN-β with AAV-hTERT-TRAIL. IFN-β staining was also seen in tumor tissue receiving AAV-hTERT-TRAIL injection alone (Fig. 7B). In contrast, no IFN-β expression was observed in tumors received PBS or AAV-hTERT-EGFP. It suggests that antitumor effect of AAV-hTERT-IFN-β on lung cancer may be a consequence of IFN-β overexpression. To understand the mechanism underlying IFN-β-induced tumor growth suppression, tumor sections were further analyzed for cell death by HE staining. The results showed that infections of AAV-hTERT-IFN-β, AAV-hTERT-TRAIL, and their combination caused profound cell death and necrosis in tumor mass, whereas the effect of combinational treatment is more obvious. No cell death was found in tumor tissue receiving PBS or AAV-hTERT-EGFP injection (Fig. 7A).

Fig. 7 Detection of cell death and IHC staining for IFN-β in tumor tissue in vivo. (A) H-E staining. Tumor sections were excised, fixed, dewaxed, followed by HE staining. Lung tumor in nude mice injected with various viruses or PBS. Tumor tissues treated with the combination showed more cell death than others groups. The arrow denotes the death of tumor cells (200×). (B) Representative micrographs of IHC staining. Tumor sections were processed as described in Materials and Methods and incubated with primary antibodies against human IFN-β. Representative images of at least 3 experiments. The expression of IFN-β in tumors as indicated with the arrows (200×).
3 Discussion
We recently demonstrated that treatment with AAV-hTERT-IFN-β not only caused tumor-specific cytotoxic effect in a panel of tumor cell lines but also effectively suppressed xenograft tumor growth either colorectal cancer or lung cancer in nude mice. Our findings suggested that IFN-β meditated by AAV under the control of hTERT promoter could inhibit tumor cell growth by inducing apoptosis[16]. Others recently have also showed that AAV-mediated IFN-β can efficaciously lead to tumor regression both in murine neuroblastoma models and even in clinical glioblastoma growth by intracerebroventricular (ICV) injection[21-22]. In this study, we characterized the expression of IFN-β and inducement of apoptosis by AAV-hTERT-IFN-β on A549 lung cancer cells and MRC5 normal cells, and found that tumor cells were more sensitive to AAV-hTERT-IFN-β-induced apoptosis and expressed IFN-β than that of normal cells. Moreover, a combined treatment of AAV-hTERTIFN-β and AAV-hTERT-TRAIL resulted in a profound tumor cytotoxic effect and apoptosis phenomenon in tumor cells.
IFNs are the multifunctional regulatory cytokine, and were discovered on the basis of their potent antiviral activities since the late 1950s[23]. Currently, all IFNs can be classified into two groups, type I (IFN-α, IFN-β, and IFN-ω) and type II (IFN-γ) according to their different cell surface receptors. Besides the antiviral effects, IFNs have become increasingly recognized for their pleiotropic biological activities, including antitumor and immunomodulatory effects in tumor therapy[9,24]. Despite acquired interesting preclinical results, there exist some limitations including short half life and systemic toxicity at high doses in the antitumor efficacy of type I IFNs[25-26]. Hence, some studies explored that virus-mediated delivery of type I IFNs was able to efficiently circumvent the pitfall and achieve long-term and significant antitumor effect[11-12]. Among these used viral vectors, AAV-mediated IFN-β delivery exhibited efficacious suppression action of tumor growth. In order to improve its tumor targeting ability, we previously used hTERT promoter to control AAV-mediated IFN-β and obtained tumor-specific IFN-β expression and antitumor effect in tumor cells and xenografts in nude mice[16].
TRAIL recently is developed as a potential therapeutic agent because it kills various tumor cells via an apoptotic cascade while spares normal cells[27]. The function of TRAIL in suppression of tumor cells and induction of apoptosis had been studied in detail by others[28]. Previous study showed that AAV-mediated TRAIL by hTERT promoter (AAV-hTERTTRAIL) induced the tumor-specific cell apoptosis and TRAIL expression[15,29]. We displayed in this study that AAV-hTERT-TRAIL transduction enhanced tumor cytotoxic and apoptotic effect of AAV-hTERT-IFN-β (Fig. 3, Fig. 5), suggesting TRAIL has intimate linkage with IFN-β. Some reported TRAIL is a key target in S-phase slowing-dependent apoptosis induced by IFN-β in cervical carcinoma cells and strongly boosted the apoptotic response of IFN-β by deregulating cell cycle[30]. Others also showed the interaction of IFN-β and TRAIL was able to induce apoptosis in ovarian carcinoma, lymphoma, multiple sclerosis, and colorectal cell through different way such as STAT1-demendent[20,31-32]. However, the synergy mechanism of the apoptotic induction and growth inhibition of tumor cells by the combination of IFN-β with TRAIL was not clear and needs to be investigated in the future studies.
It is known that the multiple, effective and tumor-specific therapeutic effect is necessary for an antitumor agent. Our preclinical study indicated that AAV-mediated IFN-β or TRAIL expression driven by hTERT promoter exhibited the remarkable antitumor efficacy. But the therapeutic effect is not ideal especially in lung cancer, and that lung cancer currently became one of the most common cause of cancer-related death, and more and more lung cancer occurred in recent years due to the environmental destroy and aggravating contamination[33]. To be interesting, the tumor-targeting strategy used here proved to be highly efficacious. We showed here that a combined injection of AAV-hTERT-IFN-β and AAV-hTERT-TRAIL in A549 lung cancer xenograft resulted in a profound inhibition in tumor growth and almost eliminated all tumor masses in nude mice (Fig. 6A), and unreported before in vivo. These findings imply the great clinical application of AAV-hTERTIFN-β in combination with AAV-hTERT- TRAIL in addition to currently available chemotherapy or radiotherapy due to their different mechanisms.
In conclusion, we showed that the IFN-β overexpression transduced by AAV-hTERT-IFN-β, and the combination of AAV-hTERT-IFN-β and AAV-hTERT-TRAIL inhibited tumor cell growth both in vitro and in vivo. The inhibitory ability of AAV-hTERT-IFN-β and AAV-hTERT-TRAIL is consistent with the apoptotic effect induced by them. The results demonstrated that either AAV-hTERT-IFN-β or AAV-hTERT-TRAIL exhibited potent antitumor activity in lung cancer subcutaneous xenograft animal model, and even their combined intratumoral injection completely suppressed the growth of tumor xenografts and remarkably improved animal survival. Consequently, these findings provided the novel tumor targeting strategy of dual gene therapy approach, which might be a potential way for clinical cancer therapy.
[1] Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther, 2006, 14(3): 316–327.
[2] Wang YG, Huang F, Cai R, et al. Targeting strategies for adeno-associated viral vector. Chin Sci Bull, 2007, 52(12): 1590–1599.
[3] Zou W, Luo C, Zhang Z, et al. A novel oncolytic adenovirus targeting to telomerase activity in tumor cells with potent. Oncogene, 2004, 23(2): 457–464.
[4] Su CQ, Wang XH, Chen J, et al. Antitumor activity of a hTERT promoter-regulated tumor-selective oncolytic adenovirus in human hepatocellular carcinoma. World J Gastroenterol, 2006, 12(47): 7613–7620.
[5] Tada H, Maron DJ, Choi EA, et al. Systemic IFN-β gene therapy results in long-term survival in mice with established colorectal liver metastases. J Clin Invest, 2001, 108(1): 83–95.
[6] Vitale G, de Herder WW, van Koetsveld PM, et al. IFN-β is a highly potent inhibitor of gastroenteropancreatic neuroendocrine tumor cell growth in vitro. Cancer Res, 2006, 66(1): 554–562.
[7] Brown JL, Barsoum J, Qin XQ. CD4+ T-helper cell-independent antitumor response mediated by murine IFN-β gene delivery in immunocompetent mice. J Interferon Cytokine Res, 2002, 22(6): 719–728.
[8] Odaka M, Wiewrodt R, DeLong P, et al. Analysis of the immunologic response generated by Ad.IFN-β during successful intraperitoneal tumor gene therapy. Mol Ther, 2002, 6(2): 210–218.
[9] Tanabe T, Kominsky SL, Subramaniam PS, et al. Inhibition of the glioblastoma cell cycle by type I IFNs occurs at both the G1 and S phases and correlates with the upregulation of p21(waf1/cip1). J Neurooncol, 2000, 48(3): 225–232.
[10] Streck CJ, Ng CY, Zhang Y, et al. Interferon-mediated anti-angiogenic therapy for neuroblastoma. Cancer Lett, 2005, 228(1/2): 163–170.
[11] He LF, Gu JF, Tang WH, et al. Significant antitumor activity of oncolytic adenovirus expressing human interferon-β for hepatocellular carcinoma. J Gene Med, 2008, 10(9): 983–992.
[12] Streck CJ, Dickson PV, Ng CY, et al. Antitumor efficacy of AAV-mediated systemic delivery of interferon-β. Cancer Gene Ther, 2006, 13(1): 99–106.
[13] Griffith TS, Stokes B, Kucaba TA, et al. TRAIL gene therapy: from preclinical development to clinical application. Curr Gene Ther, 2009, 9(1): 9–19.
[14] Wang YG, Wang JH, Zhang YH, et al. Antitumor effect of a novel adeno-associated virus vector targeting to telomerase activity in tumor cells. Acta Biochim Biophys Sin, 2004, 36(7): 492–500.
[15] Wang Y, Huang F, Cai H, et al. Potent antitumor effect of TRAIL mediated by a novel adeno-associated viral vector targeting to telomerase activity for human hepatocellular carcinoma. J Gene Med, 2008, 10(5): 518–526.
[16] He LF, Wang YG, Xiao T, et al. Suppression of cancer growth in mice by adeno-associated virus vector-mediated IFN-β expression driven by hTERT promoter. Cancer Lett, 2009, 286(2): 196–205.
[17] Liu XY, Gu JF. Targeting gene-virotherapy of cancer. Cell Res, 2006, 16(1): 25–30.
[18] Chawla-Sarkar M, Leaman DW, Jacobs BS, et al. IFN-β pretreatment sensitizes human melanoma cells to TRAIL/Apo2 ligand-induced apoptosis. J Immunol, 2002, 169(2): 847–855.
[19] Leaman DW, Chawla-Sarkar M, Jacobs B, et al. Novel growth and death related interferon-stimulated genes (ISGs) in melanoma: greater potency of IFN-β compared with IFN-α2. J Interferon Cytokine Res, 2003, 23(12): 745–756.
[20] Morrison BH, Tang Z, Jacobs BS, et al. Apo2/TRAIL induction and nuclear translocation of inositol hexakisphosphate kinase 2 during IFN-β-induced apoptosis in ovarian carcinoma. Biochem J, 2005, 385(Pt2): 595–603.
[21] Streck CJ, Dickson PV, Ng CY, et al. Adeno-associated virus vector-mediated systemic delivery of IFN-β combined with low-dose cyclophosphamide affects tumor regression in murine neuroblastoma models. Clin Cancer Res, 2005, 11(16): 6020–6029.
[22] Meijer DH, Maguire CA, Leroy SG, et al. Controlling brain tumor growth by intraventricular administration of an AAV vector encoding IFN-β. Cancer Gene Ther, 2009, 16(8): 664–671.
[23] Pfeffer LM, Dinarello CA, Herberman RB, et al. Biological properties of recombinant α-interferons: 40th anniversary of the discovery of interferons. Cancer Res, 1998, 58(12): 2489–2499.
[24] Dunn IS, Haggerty TJ, Kono M, et al. Enhancement of human melanoma antigen expression by IFN-β. J Immunol, 2007, 179(4): 2134–2142.
[25] Fierlbeck G, Ulmer A, Schreiner T, et al. Pharmacodynamics of recombinant IFN-β during long-term treatment of malignant melanoma. J Interferon Cytokine Res, 1996, 16(10): 777–781.
[26] Einhorn S, Grander D. Why do so many cancer patients fail to respond to interferon therapy? J Interferon Cytokine Res, 1996, 16(4): 275–281.
[27] Kruyt FA. TRAIL and cancer therapy. Cancer Lett, 2008, 263(1): 14–25.
[28] Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene, 2008, 27(48): 6207–6215.
[29] Zhang Y, Ma H, Zhang J, et al. AAV-mediated trail gene expression driven by hTERT promoter suppressed human hepatocellular carcinoma growth in mice. Life Sci, 2008, 82(23/24): 1154–1161.
[30] Vannucchi S, Chiantore MV, Fiorucci G, et al. TRAIL is a key target in S-phase slowing-dependent apoptosis induced by interferon-β in cervical carcinoma cells. Oncogene, 2005, 24(15): 2536–2546.
[31] Choi EA, Lei H, Maron DJ, et al. STAT1-dependent induction of tumor necrosis factor-related apoptosisinducing ligand and the cell-surface death signaling pathway by interferon β in human cancer cells. Cancer Res, 2003, 63(17): 5299–5307.
[32] Wandinger KP, Lunemann JD, Wengert O, et al. TNF-related apoptosis inducing ligand (TRAIL) as a potential response marker for interferon-β treatment in multiple sclerosis. Lancet, 2003, 361(9374): 2036–2043.
[33] Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2008: a review of current american cancer society guidelines and cancer screening issues. CA Cancer J Clin, 2008, 58(3): 161–179.
