Stanniocalcin-1 Detection of Peripheral Blood in Patients with Colorectal Cancer
2010-01-08PingpingWuPengWuPeilinHuangQiqiangLongXiaodongBu
Ping-ping Wu,Peng Wu,Pei-lin Huang*,Qi-qiang Long,Xiao-dong Bu
1Department of Oncology,Jiangsu Cancer Hospital; 2Department of Pathology,Southeast University Medical College,Nanjing 210009,China
INTRODUCTION
Colorectal cancer (CRC)is one of the most common and deadly malignancies worldwide.Even though primary foci and metastases are ablated and routine pathological detection is judged negative,many patients eventually die of the metastasis and recurrence of cancer.The reason for this is tumor micrometastasis: during the prophase of malignancy,a few tumor cells from the original region may disseminate into lymph,marrow,blood and distant organs and survive without clinical representation, and undetected by normal methods[1].Micrometastasis of tumor cells in peripheral blood is an important initiative step for metastasis and tumor recurrence; its existence as a significant but compelling prognostic factor has been increasingly reported[2].Although detection of micrometastasis could greatly improve the survival rate of patients,there is currently no appropriate biomarker for practical clinical use.
Stanniocalcin-1 (STC-1) is a kind of glycoprotein hormone,first found in bony fish and later in human and mammals,with a highly conserved homology.Its primary function in fish is to prevent hypercalcemia and stimulate phosphate reabsorption[3]; in mammals,it seems to play a role in many cellular processes[4-6].Intriguingly,STC-1 has a close association with the occurrence and development of human carcinomas[7].Though negative in the marrow and blood of cancer-free humans,STC-1 expression levels are universally much higher in tumor tissues and cancer cell lines than in corresponding normal tissues,which suggests that STC-1 may be useful as a tumor marker[8,9].Recently,RT-PCR has been found to be a sensitive method of detecting the expression of tumor-specific mRNA in peripheral blood,marrow and lymph[10,11],allowing the detection of a very small number of malignant cells from a sample of~107monocytes.Thus,we detected the expression of STC-1 mRNA using RT-PCR in the peripheral blood of CRC patients,and investigated its relation with CRC micrometastasis and prognosis.
PATIENTS AND METHODS
Patients
A total of 78 peripheral blood samples from patients with CRC were obtained at the Affiliated Zhongda Hospital of Southeast University between September 2002 and January 2004.None received prior treatment,such as surgery,chemotherapy or radiotherapy.The age of the patients ranged from 33 to 78 with a median age of 61 and an average age of 61.3.Histological classification was conducted according to the 2000 WHO classification system for tumors of digestive system,and tumor stage was determined according to the 2002 TNM staging guidelines suggested by the American Joint Committee on Cancer and the Union International Contre le Cancer (UICC).Blood samples from 14 healthy volunteers and 19 patients with inflammatory colorectal diseases were collected as a negative control,and 15 fresh tumor tissues from gastrointestinal cancer patients were obtained as a positive control.Ethical approval from the hospital,and fully informed consent from all patients was obtained prior to sample collection.The follow-up data were available for 63 patients,and follow-up periods for survivors ranged from 3 to 60 months,with a median follow-up time of 42 months.
Specimen Treatment
Five milliliters of peripheral blood was collected from each patient described above on the next day after admission.The blood samples were kept in a 4°C refrigerator with the presence of anticoagulant for no longer than 2 h.The peripheral blood mononuclear cells (PBMNCs)were isolated using Lymphocyte Separation Medium (Sigma,St.Louis,USA)according to the manufacturer’s protocol.PBMNCs and tumor tissues were stored at -80°C until use.
RNA Extraction and RT- PCR Reaction
Total RNA extraction from PBMNCs or tumor tissues was performed using Trizol reagent(Invitrogen, Carlsbad, CA, USA).Reverse transcription reaction (RT)was performed using 2μg of total RNA with a first strand cDNA kit(Takara,Shiga,Japan).Polymerase chain reaction(PCR)was performed in 25μl final volume containing 2.5μl PCR buffer,5μl cDNA template,20 pM of each primer and 0.75U Taq DNA polymerase (Takara).The primers and PCR amplification conditions are listed in Table 1.PCR products were analyzed by electrophoresis on 1.8%agarose gels containing ethidium bromide.
Statistical Analysis
Statistical analyses were performed using SPSS 12.0 statistical software (SPSS Inc.,Chicago,IL).Differences in frequency were assessed by Chi-square test or Fisher’s exact test.The cum survival curves were calculated using the Kaplan-Meier method and differences in the survival rates were analyzed using the log-rank test.P<0.05 was considered statistically significant.
RESULTS
STC-1 mRNA Expression Profile
Of the 78 blood samples collected from primary CRC patients,33 (42.31%)showed positive expressions of STC-1 mRNA.All 15 gastrointestinal tumor tissues were positive for STC-1 mRNA.In contrast,all 19 blood samples collected from healthy donors and all 14 samples from patients with inflammatory gastrointestinal disease were negative(P<0.001).A typical agarose gel electrophoresis picture interpreting the RT-PCR results is shown in Figure 1.
Clinicopathological Correlation
The relationship between STC-1 mRNA expression status and clinicopathological features was analyzed according to sex,age,histological type,cellular differentiation,TNM stages,lymph metastasis and distant metastasis.As indicated in Table 2,STC-1 mRNA expression status was seen preferentially in the cases of stage III/IV (P=0.009)or harboring distant metastasis (P=0.024),but there was no correlation with patient gender,age,histological type and lymph metastasis.Although positive rates of STC-1 mRNA were relative higher in poorly differentiated or undifferentiated tumors than in well and moderately differentiated tumors,no statistical significance was found.
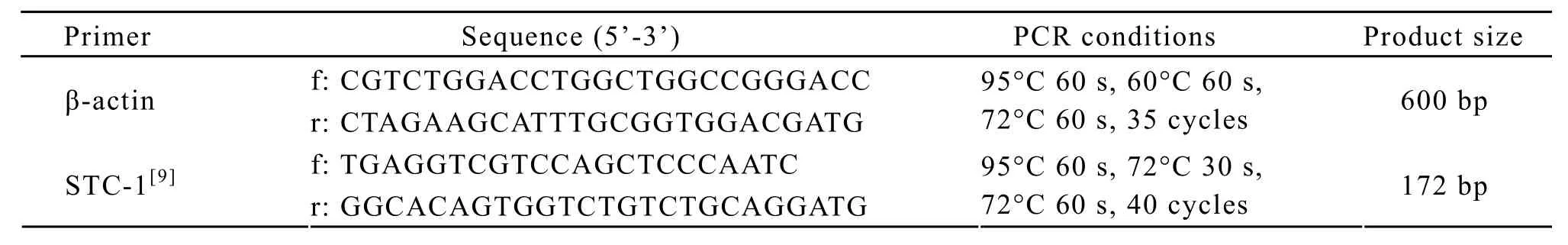
Table 1.List of primer sequences and amplification conditions
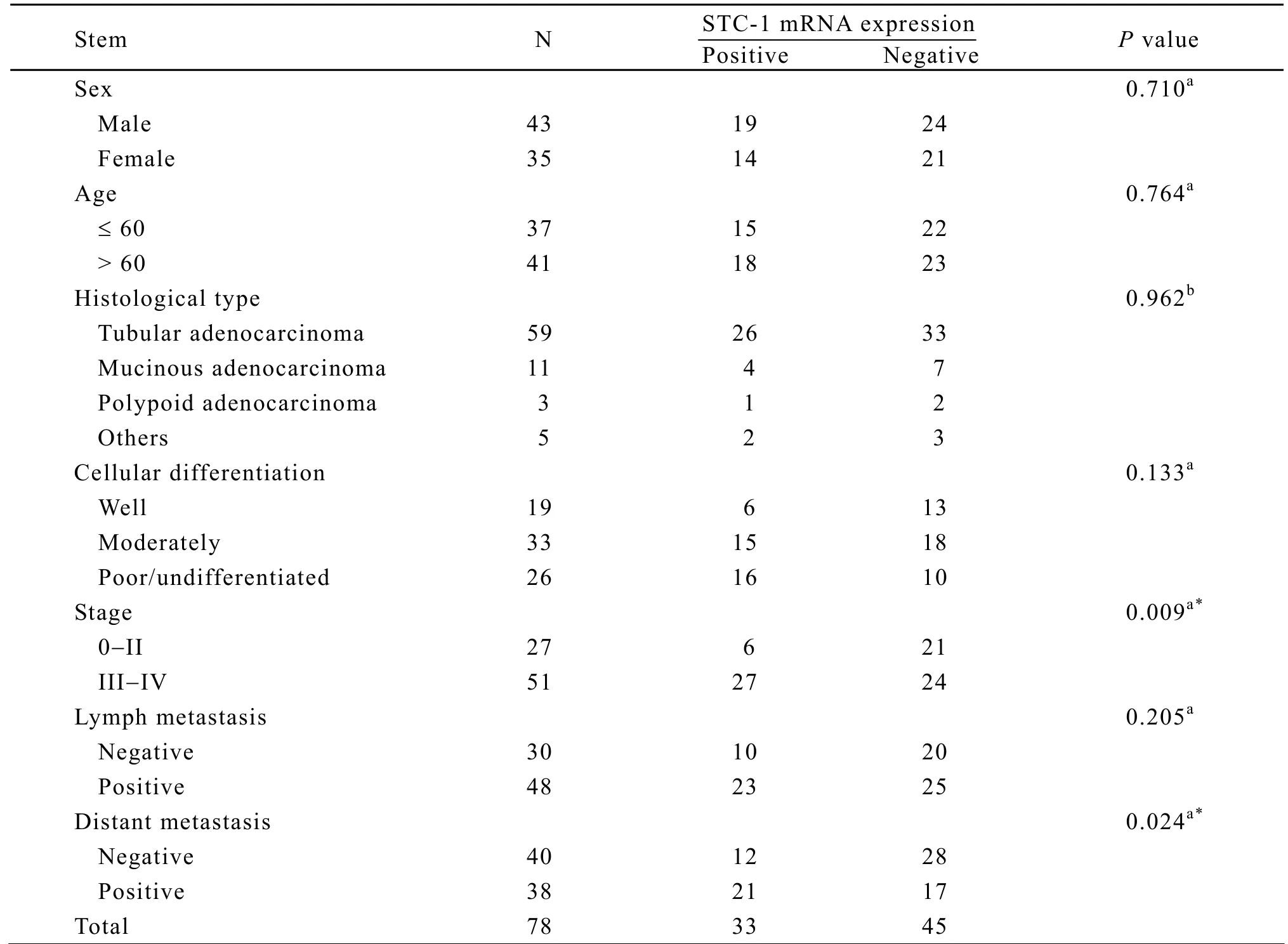
Table 2.Association between the expression of STC-1 mRNA and clinicopathological features
STC-1 Expression and Survival Analysis
Univariate survival analyses were performed to investigate a possible prognostic impact of STC-1 in colorectal cancers.There was a significant difference in 5-year overall survival between patients showed positive or negative STC-1 mRNA expression in peripheral blood (P=0.030; Figure 2).Furthermore,the median survival in the positive group was 36 months,whereas in the negative group was 45 months (P=0.043).These results indicated that STC-1 expression in blood was an independent unfavorable prognostic factor for CRC.
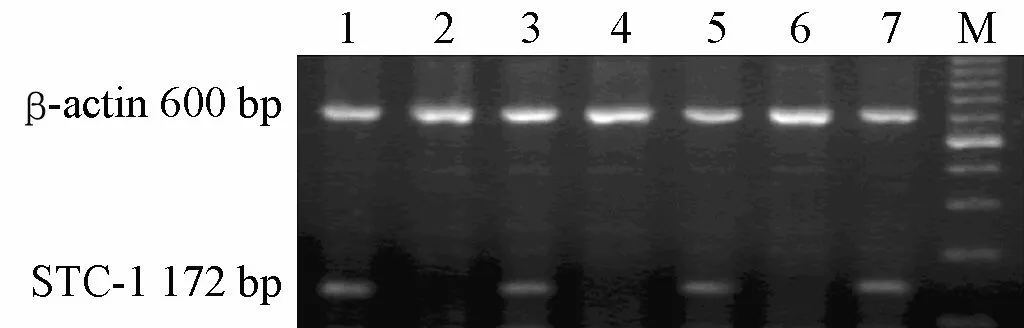
Figure 1.RT-PCR analysis of STC-1 mRNA expression in peripheral blood.Lane 1: Result for tumor tissue.Lane 2: Blood of healthy volunteer.Lanes 3-7: Blood samples of patients with colorectal cancer.Lane M:100 bp DNA ladder marker.β-actin was used as an internal control.
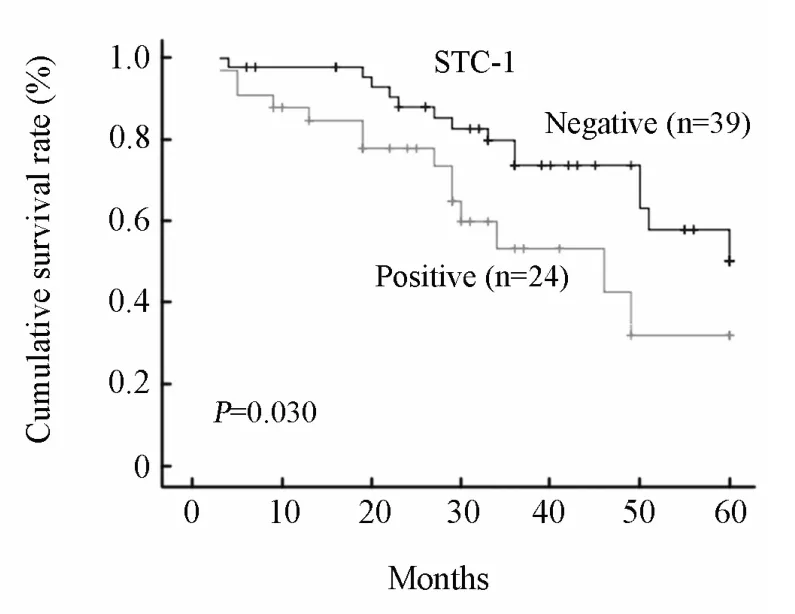
Figure 2.The 5-year overall survival of CRC patients according to STC-1 expression.Survival curves of CRC patients with positive and negative STC-1 transcripts in blood were plotted by Kaplan-Meier method and their difference was evaluated by the log-rank test.There was significant difference in overall survival between the two groups (P=0.030).
DISCUSSION
Stanniocalcin-1 gene is widely expressed in a paracrine or autocrine fashion in various tissues,including the heart,lung,liver,adrenal glands,kidneys,prostate and ovaries[4,12],but did not do so in the peripheral blood,except in pregnancy[13].Niu,et al.suggested that a special elimination mechanism might be in the blood,leading to the degradation of STC-1[12].
Mounting evidences suggest that altered expression of the STC-1 may have a role in human cancer.Enhanced STC-1 gene expression has been found in hepatocellular,colorectal,breast cancer,and medullary thyroid cancer[7,9,14].In colon cancer,the levels of STC-1 expression were at least 10-fold higher than that in normal mucosa[15].It has also been found that STC-1 mRNA transcripts rise remarkably in the blood of tumor patients[16].An analysis of blood samples obtained from 11 patients with hepatocellular carcinoma before,after and during hepatectomy showed STC-1 mRNA expression in 8 out of 11 patients at least one time,while normal donors’ blood samples(n=31)were all negative for STC-1 mRNA expression[9].The possible use of STC-1 expression levels for the diagnosis of breast cancer and leukemia has also been proposed[17,18].
Our results indicated that the positive rate of STC-1 mRNA,detected by RT-PCR in the peripheral blood from 78 patients with colorectal cancer in this experiment,was 42.31% (33/78),while in the control samples from 14 healthy volunteers and 19 patients with inflammatory gastrointestinal diseases, none showed the expression of STC-1 mRNA.The sensitivity and specificity were satisfactory compared with the commonly used biomarkers of colorectal cancer,such as carcinoembryonic antigen (CEA),or CA19-9[19],thus STC-1 mRNA detected in the blood may be a promising molecular marker for colorectal cancer.Even in patients who had no lymph metastasis or distant metastasis,the positive rates of STC-1 transcripts were as high as 33.3%and 30%,respectively.Because colorectal cancer is a systemic disease,when a malignancy is tiny and still in the suspect state,cancer cells may enter the bloodstream.Clinicians should therefore consider the possibility of micrometastasis if STC-1 mRNA transcripts are detected in the peripheral blood.
Furthermore,we found that patients in stages III-IV had higher positive rates of STC-1 expression than those in stages I-II,and STC-1 expression in the peripheral blood was significantly related with distant metastasis and lower 5-year overall survival,while not related with the sex,age,histological type,cellular differentiation or lymph metastasis.We therefore thought that checking STC-1 mRNA transcripts in the peripheral blood possibly not only warn of malignant tumors,but also hint of advanced stage and unfavorable prognosis.Our study found no statistical significance with regard to cellular differentiation and lymph metastasis,which may be due to small sample size.
Regrettably,little is known about STC-1 signaling and its functions in cancer progression,although roles in calcium and phosphate homeostasis and ovarian function have been suggested[20,21].However,STC-1 is up-regulated in endothelial cells undergoing tubulogenesis[22],and intensely expressed in the vasculature of colon carcinomas.STC-1 expression has also been found to be induced by vascular endothelial growth factor[23]and hepatocyte growth factor[24].Moreover,STC-1 regulates gene expression in cultured endothelial cells and is detected on the apical surface of endothelial cells in vivo[25],suggesting this novel glycoprotein might play an important role in one or more of the processes associated with angiogenesis.A recent study shows that STC-1 targets the mitochondrial matrix to stimulate electron transport,uncouple oxidative phosphorylation and enhance mitochondrial calcium accumulation[26].Thus the elevated expression of STC-1 during angiogenesis may play an additional role in the metabolic requirements of endothelial cells and other cells involved in the formation of new blood vessels.STC-1 is also proposed to be a tumor suppressor gene in relation to apoptosis.This putative role is supported by a high rate of loss of heterozygosity at the chromosomal loci at 8p11.2-p21 in many cancer cases,where STC-1 is localized[27].In addition,STC-1 was found to be regulated by p53[28]and NFκB[29],which are known to be involved in the regulation of cell survival.Together with other published reports[6,7,30],the accumulated evidence implicates STC-1 in cellular apoptotic processes in human carcinogenesis.Recent evidence has shown that STC-1 expression is markedly increased in hypoxia,a common phenomenon that occurs in regions of most solid human tumors in the latter stages of carcinogenesis,and endogenous hypoxiainducible factor 1 induces the expression of STC-1 in the condition of anoxia[31],suggesting that STC-1 could prompt cancer cells to adapt to hypoxic environments.
In summary,we found that detection of STC-1 mRNA in the blood may be a useful molecular marker for colorectal cancer,particularly in the assessment of micrometastasis and prediction of prognosis.However,the mechanism of STC-1 function is not yet clear,suggesting that additional study of the physiological role of STC-1 in both normal and cancer cells is needed.
[1]Wikman H,Vessella R,Pantel K.Cancer micrometastasis and tumour dormancy[J].APMIS 2008;116:754-70.
[2]Feezor RJ,Copeland EM 3rd,Hochwald SN.Significance of micrometastases in colorectal cancer[J].Ann Surg Oncol 2002; 9: 944-53.
[3]Wagner GF, Jaworski EM, Haddad M.Stanniocalcin in the seawater salmon: structure,function,and regulation[J].Am J Physiol 1998;274:R1177-85.
[4]Ishibashi K,Imai M.Prospect of a stanniocalcin endocrine/paracrine system in mammals[J].Am J Physiol Renal Physiol 2002; 282:F367-75.
[5]Li K,Dong D,Yao L,et al.Identification of STC-1 as an beta-amyloid activated gene in human brain microvascular endothelial cells using cDNA microarray[J].Biochem Biophys Res Commun 2008; 376:399-403.
[6]Wu S,Yoshiko Y,De Luca F.Stanniocalcin 1 acts as a paracrine regulator of growth plate chondrogenesis[J].J Biol Chem 2006; 281:5120-7.
[7]Chang AC,Jellinek DA,Reddel RR.Mammalian stanniocalcins and cancer[J].Endocr Relat Cancer 2003; 10:359-73.
[8]Joensuu K,Heikkilä P,Andersson LC.Tumor dormancy: Elevated expression of stanniocalcins in late relapsing breast cancer[J].Cancer Lett 2008; 265:76-83.
[9]Fujiwara Y,Sugita Y,Nakamori S,et al.Assessment of stanniocalcin-1 mRNA as a molecular market for micrometastases of various human cancers[J].Int J Oncol 2000; 16:799-804.
[10]Yang HW,Yang NW,Cao J,et al.Detection of SBEM-mRNA in peripheral blood of patients with breast cancer and its clinical significance[J].Chin J Cancer Res 2006; 18:294-8.
[11]Huang P,Wang J,Guo Y,et al.Molecular detection of disseminated tumor cells in the peripheral blood in patients with gastrointestinal cancer[J].J Cancer Res Clin Oncol 2003; 129:192-8.
[12]De Niu P,Radman DP,Jaworski EM,et al.Development of a human stanniocalcin radioimmunoassay: serum and tissue hormone levels and pharmacokinetics in the rat[J].Mol Cell Endocrinol 2000; 162:131-44.
[13]Deol HK,Varghese R,Wagner GF,et al.Dynamic regulation of mouse ovarian stanniocalcin expression during gestation and lactation[J].Endocrinology 2000; 141:3412-21.
[14]McCudden CR,Majewski A,Chakrabarti S,et al.Co-localization of stanniocalcin-1 ligand and receptor in human breast carcinomas[J].Mol Cell Endocrinol 2004; 213:167-72.
[15]Gerritsen ME,Soriano R,Yang S,et al.In silico data filtering to identify new angiogenesis targets from a large in vitro gene profiling data set[J].Physiol Genomics 2002; 10:13-20.
[16]Koide Y,Sasaki T.Stanniocalcin-1 (STC-1)as a molecular marker for human cancer[J].Rinsho Byori 2006; 54:213-20.
[17]Wascher RA,Huynh KT,Giuliano AE,et al.Stanniocalcin-1: a novel molecular blood and bone marrow marker for human breast cancer[J].Clin Cancer Res 2003; 9:1427-35.
[18]Tohmiya Y,Koide Y,Fujimaki S,et al.Stanniocalcin-1 as a novel marker to detect minimal residual disease of human leukemia[J].Tohoku J Exp Med 2004; 204:125-33.
[19]Yamashita K,Watanabe M.Clinical significance of tumor markers and an emerging perspective on colorectal cancer[J].Cancer Sci 2009; 100:195-9.
[20]Filvaroff EH,Guillet S,Zlot C,et al.Stanniocalcin 1 alters muscle and bone structure and function in transgenic mice[J].Endocrinology 2002; 143:3681-90.
[21]Paciga M,Watson AJ,DiMattia GE,et al.Ovarian stanniocalcin is structurally unique in mammals and its production and release are regulated through the luteinizing hormone receptor[J].Endocrinology 2002; 143:3925-34.
[22]Kahn J,Mehraban F,Ingle G,et al.Gene expression profiling in an in vitro model of angiogenesis[J].Am J Pathol 2000; 156:1887-900.
[23]Wary KK,Thakker GD,Humtsoe JO,et al.Analysis of VEGF-responsive genes involved in the activation of endothelial cells[J].Mol Cancer 2003; 2:25.
[24]Zlot C,Ingle G,Hongo J,et al.Gerritsen,Stanniocalcin 1 is an autocrine modulator of endothelial angiogenic responses to hepatocyte growth factor[J].J Biol Chem 2003; 278:47654-9.
[25]Chakraborty A,Brooks H,Zhang P,et al.Stanniocalcin-1 regulates endothelial gene expression and modulates transendothelial migration of leukocytes[J].Am J Physiol Renal Physiol 2007; 292:F895-904.
[26]Ellard JP,McCudden CR,Tanega C,et al.The respiratory effects of stanniocalcin-1 (STC-1)on intact mitochondria and cells: STC-1 uncouples oxidative phosphorylation and its actions are modulated by nucleotide triphosphates[J].Mol Cell Endocrinol 2007; 264:90-101.
[27]Macartney-Coxson DP,Hood KA,Shi HJ,et al.Metastatic susceptibility locus,an 8p hot-spot for tumour progression disrupted in colorectal liver metastases: 13 candidate genes examined at the DNA,mRNA and protein level[J].BMC Cancer 2008; 8:187.
[28]Lai KP,Law AY,Yeung HY,et al.Induction of stanniocalcin-1 expression in apoptotic human nasopharyngeal cancer cells by p53[J].Biochem Biophys Res Commun 2007; 356:968-75.
[29]Law AY,Lai KP,Lui WC,et al.Histone deacetylase inhibitor-induced cellular apoptosis involves stanniocalcin-1 activation[J].Exp Cell Res 2008; 314:2975-84.
[30]McCudden CR,James KA,Hasilo C,et al.Characterization of mammalian stanniocalcin receptors.Mitochondrial targeting of ligand and receptor for regulation of cellular metabolism[J].J Biol Chem 2002; 277:45249-58.
[31]Yeung HY,Lai KP,Chan HY,et al.Hypoxiainducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells[J].Endocrinology 2005; 146:4951-60.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Methylation and Demethylation of Ink4 Locus in Cancer Development
- Apoptotic B16-F1 Cells Coated with Recombinant Calreticulin Mediated Anti-tumor Immune Response in Mice
- Polymorphisms of UGT1A7 and XRCC1 are Associated with an Increased Risk of Hepatocellular Carcinoma in Northeast China
- Risk Factors of Precancerous Gastric Lesions in A Population at High Risk of Gastric Cancer
- Promoter Hypermethylation of KiSS-1 Gene in Gastric Cancer
- Recurrent Patterns and Factors Involved in Node-negative Advanced Gastric Cancer
