Risk Factors of Precancerous Gastric Lesions in A Population at High Risk of Gastric Cancer
2010-01-08JianLiuLiPingSunYueHuaGongYuanYuan
Jian Liu,Li-Ping Sun,Yue-Hua Gong,Yuan Yuan
Cancer Institute,The First Affiliated Hospital,China Medical University,Shenyang 110001
INTRODUCTION
Gastric cancer is an important cause of mortality and has been predicted to be the eighth leading cause of all deaths worldwide in the year 2010[1-3].In China,GC is still recognized as one of the leading cause of cancer death[4]although its mortality have been decreasing over the years in many countries due to the changing dietary habits and lifestyles.Evidences from pathology and epidemiology studies has been provided for a human model of gastric carcinogenesis with the following sequential stages: chronic gastritis;atrophy; intestinal metaplasia; and dysplasia[5].These results indicate that gastric carcinogenesis is a multistep process,and chronic gastritis; atrophy;intestinal metaplasia; and dysplasia are deemed to be the precancerous gastric lesions of gastric cancer.To slow the progression of precancerous gastric lesions may reduce the incidence of gastric cancer.Most studies showed that the incidence of precancerous gastric lesions is related to dietary factors and lifestyle[6-8].If these relative factors were eliminated or decreased,the incidence and mortality of precancerous gastric lesions would be lowered,and then,the incidence and mortality of gastric cancer may be decreased.Thus,it is very important to investigate the risk factors of these precancerous gastric lesions and find an effective comprehensive preventive method for gastric cancer.
Zhuanghe County,lying in the coastal area of Eastern-Liaoning peninsula,is an area of high morbidity of gastric cancer in north China.The objective of this study was to investigate the risk factors of clinical outcomes,like gastritis,gastric ulcer,and dysplasia in suspected high risk peoples from Zhuanghe County,taking into account their different life habits,such as alcohol ingestion,and smoking habit.
MATERIALS AND METHODS
Three villages were selected randomly for the investigation.Persons with history of gastric illness,family history of gastric cancer and older than thirty-five years were selected as suspected high risk population from selected villages according to the epidemiological survey in the two-round screening.Clinical epidemiological investigation, serum pepsinogen monitoring,gastroscopic biopsies and histopathological examination were employed for the screening.Endoscopic and clinicopathological examinations was performed for observing the entire stomach.Experienced endoscopists performed each examination without knowledge about the serological data on the study subjects.Gastric mucosa was examined,and 4 biopsy specimens were obtained from the body,angulus,antrum and lesions,respectively.The biopsies were routinely bathed in 95% alcohol,embedded in paraffin,then sectioned and stained in each local center.The stained sections were evaluated independently by two gastrointestinal pathologists.Each subject was assigned a global diagnosis based on the 4 specimens.Microscopic findings were assessed according to the consensus on chronic gastritis at the national symposium or in combination with the visual analog scale of the updated Sydney System.Finally,1179 persons without gastric cancer diseases were further investigated and grouped as high risk population of gastric cancer.All study investigators and staff members successfully completed a training program that oriented them both to the aims of the study and to the specific tools and methodologies employed.At the training sessions,interviewers were given detailed instructions on administration of the study questionnaire.This questionnaire includes: general conditions (age,sex,occupation,marriage,culture level,number of people in family and income et al.);habit of bite and sup (smoking,drinking,history of gastric illness and history of family gastric cancer and so on).Daily salt intake was measured by investigating the total amount of salt consumed by a family for 1 year and dividing this value by the number of family member × 365.
Statistical Analysis
Continuous variables were given as the±s and categorical variables as the percentage in each subgroup.Associations between categorical variables were tested by the use of contingency tables and the χ2test or Fisher test.Comparisons between continuous variables between groups were performed by Student’s t-test.Multivariate logistic regression analyses were used to test significant determinants of hypertension status.All data analyses were conducted using SAS 8.12 (Statistics/Data Analysis)software.A two-tailed probability value of <0.05 was considered to be statistically significant.
RESULTS
A total of 1179 subjects from 35 to 85 years old were included in this study.The mean age of the subjects was 52.53 (±7.79)years and 558 were male.Overall,98.81% (1165/1179)of the suspected high risk subjects had precancerous gastric lesions.The overall prevalence of precancerous gastric lesions was slightly higher among men (99.10%)than that among women (98.55%),but the difference was not statistically significant.The constituent ratio of types of precancerous gastric lesions between genders was significantly different (Table 1).With the increase of age,there was a decrease trend in superficial gastritis(Ζ=3.23,P<0.001),and an increasing trend in atrophic gastritis (Ζ=2.02,P=0.02)(Table 1).For all the participants,the prevalence of gastric epithelial dysplasia was higher among subjects with education level ≥ junior college.Workers and subjects in a family containing more than 4 members were at greater risk of developing supreficial gastritis and superficial atrophic gastritis (χ2=6.20,P=0.013)(Table 1).Education levels ≥Junior College was also the risk factors for the dysplasia.In contrast,the workers and more peoples in family (>4)were belong to the protective factors against superficial atrophic gastritis and Superficial gastritis,respectively.Marriage status and family income were not related to the gastric diseases risk in the present study.
Table 2 shows the interrelation between gastric diseases and smoking or drinking.The prevalence of atrophic gastritis,gastric ulcer and gastric epithelial dysplasia was higher in the smokers than no-smokers(χ2=11.49,P=0.043)(Table 2).And,we noted a significant dose-risk relationship existed between the daily consumed cigarettes,smoking duration and the gastric ulcer and gastric epithelial dysplasia (Figure 1 and Figure 2).Drinkers were apt to suffer from atrophic gastritis compared with non-drinkers,and the prevalence of atrophic gastritis and gastric ulcer among drinkers were 17.57% and 13.81%,respectively,which were higher than that among no-drinking groups.Also,the association of drinking and atrophic gastritis or gastric ulcer increased in strength with the increase of daily consumed alcohol and drinking duration (Figure 3 and Figure 4).
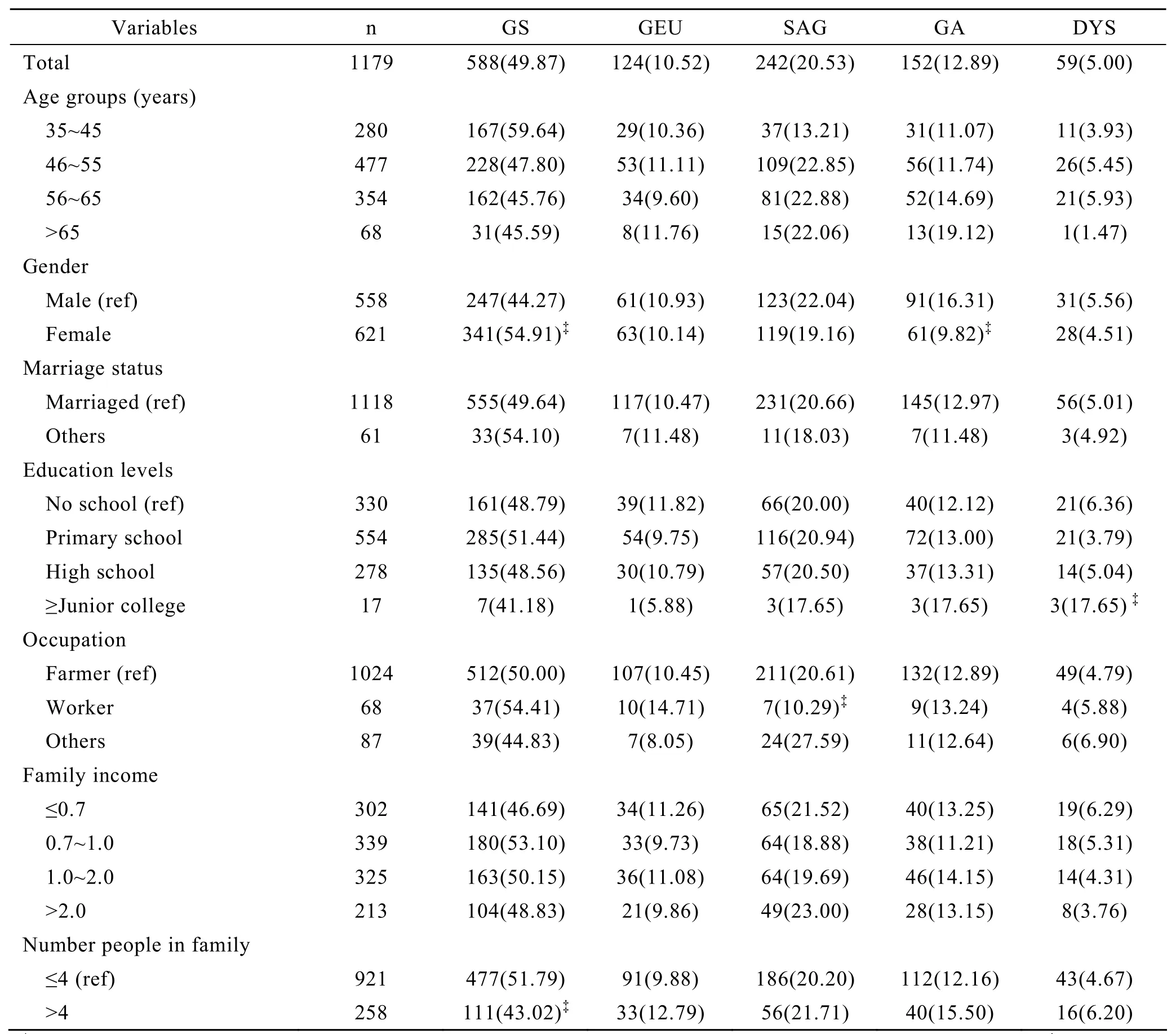
Table 1.Distribution of histopathology of gastric diseases among subjects,n(%)*
The relationship between the dietary habits and gastric diseases are shown in Table 3.Among the subjects whose salt intake was >6g,the prevalence of gastric ulcer increased with the increase in the intake of table salt (P<0.05),however,the effects of the other life-type factors such as drinking water type,pickled food intake,milk and meat intake on precancerous gastric lesions were not significantly.Unconditional logistic analyses were made by a backward elimination approach to select a possible best subset of risk factors for gastric diseases.After adjusted the effects of the other factors,the results showed that garlic eating was shown to confer protection against the development of gastric ulcer(OR=0.55,95% CI: 0.33-0.92).Subjects liking deepfry food have a higher risk of developing gastric epithelial dysplasia,the odds ratio was 1.78 (95%CI:1.01-3.12).
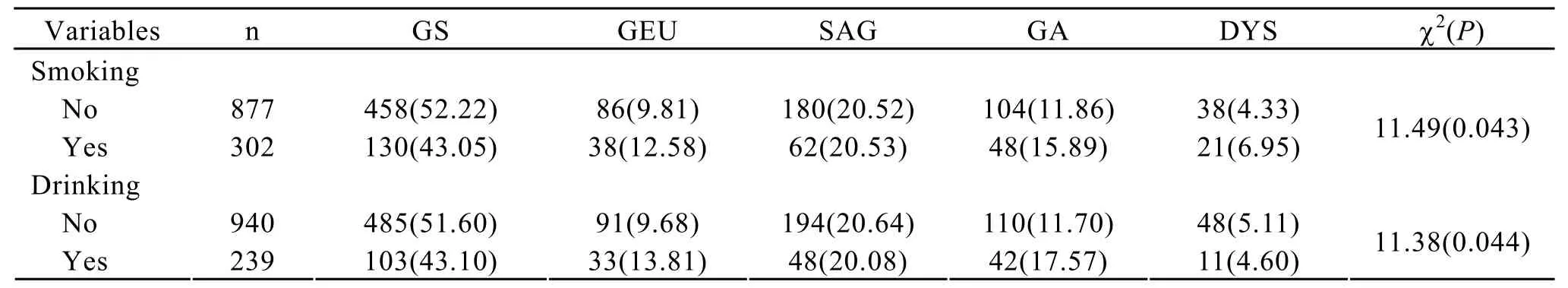
Table 2.Effects of smoking and drinking on the distribution of histopathology of gastric diseases among subjects,n(%)*
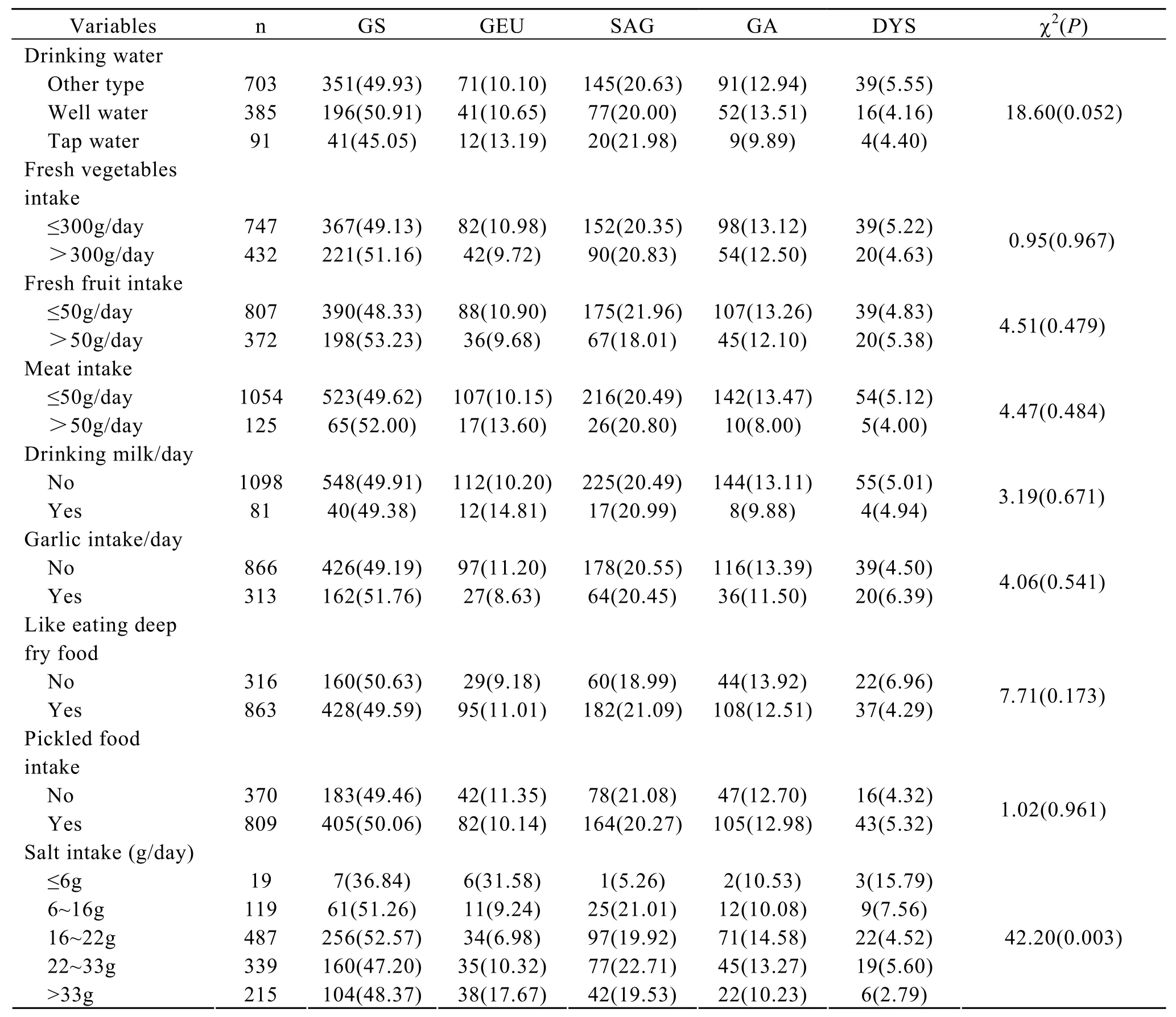
Table 3.Potential risk or protective factors for gastric diseases among subjects,n(%)*
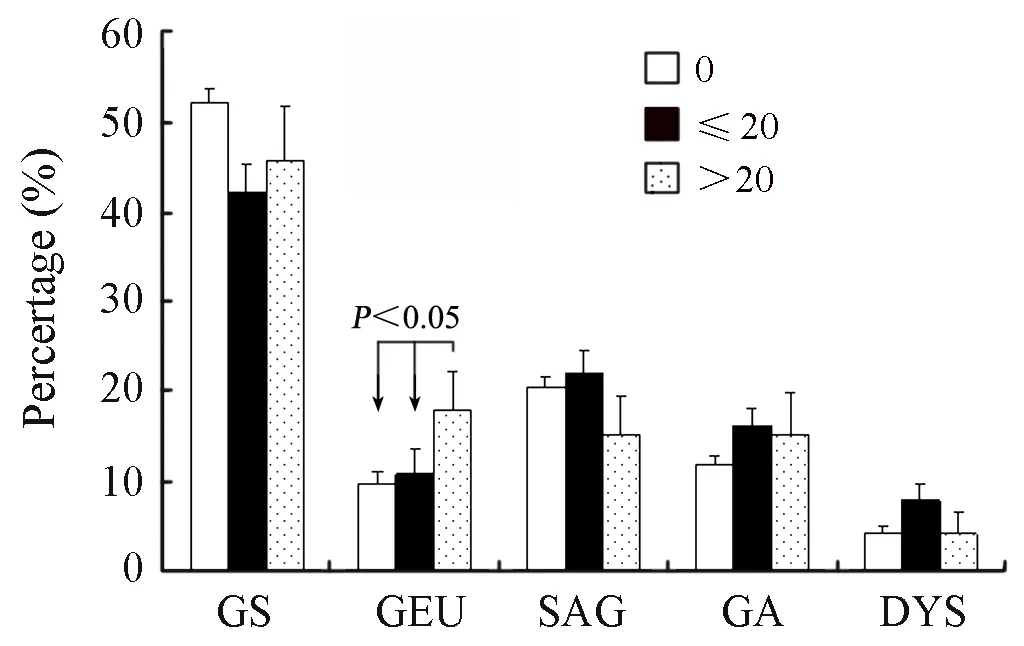
Figure 1.The interrelation between daily consumed cigarettes and gastric diseases among subjects (P±Sp),GS=Superficial gastritis; GEU=Gastric ulcer; SAG=Superficial atrophic gastritis; GA=Atrophic gastritis; DYS=Dysplasia.
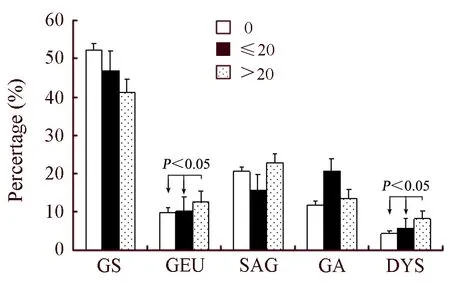
Figure 2.The interrelation between smoking duration (years)and gastric diseases among subjects (P±Sp),GS=Superficial gastritis; GEU=Gastric ulcer; SAG=Superficial atrophic gastritis; GA=Atrophic gastritis; DYS=Dysplasia.
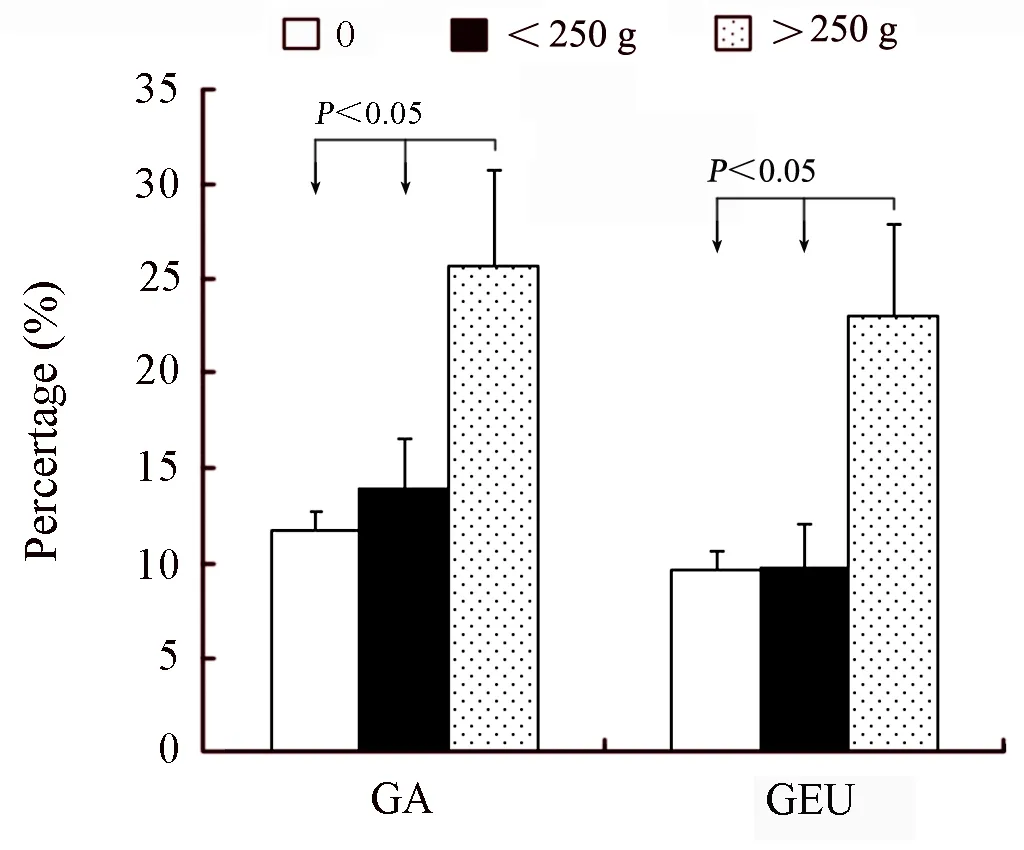
Figure 3.The interrelation between daily consumed alcohol and gastric diseases among subjects (P±Sp),GS=Superficial gastritis; GEU=Gastric ulcer; SAG=Superficial atrophic gastritis; GA=Atrophic gastritis; DYS=Dysplasia.
DISCUSSION
During the recent decades,the incidence of gastric cancer has been declining globally[9].It is believed that the main reasons of the declining are in prevention not therapy.In cancer prevention,the targeting of precancerous lesions has been recognized as the most promising method.A longitudinal study[10]in Linqu County of China found that the RR(relative risks)for subsequent development of gastric cancer were 104 (95%CI=9.7 to 999)for subjects with moderate or severe dysplasia,29 (95% CI=3.9 to 219)for subjects with mild dysplasia or deep intestinal metaplasia,and 17 (95% CI=1.5 to 202)for subjects with superficial intestinal metaplasia,all compared with subjects with superficial gastritis or chronic atrophic gastritis.These results,along with the histopathologic evidence for the multistep nature of gastric carcinogenesis[12],suggest that interventions that slow the progression of precancerous gastric lesions may reduce the incidence of gastric cancer.However,little attention has been paid to the risk factors of precancerous gastric lesions,especially in rural China where there is high prevalence of precancerous gastric lesions[12].
In our study,subjects with education levels ≥junior college were more suffered from gastric dysplasia than others.A possible reason might be that,in rural China,subjects with higher education bear more burden and stress.The relation between stress and peptic ulcer disease has been well established[13].In a longitudinal study of adults in the United States,after adjustment for other factors,gastric diseases were more likely to develop in those who perceived themselves as stressed than in those who did not[14].
Cigarette smoking and alcohol consumption have been variously implicated as risk factors for precancerous gastric lesions.As in other studies[15-17],we found both gastric ulcer and dysplasia disease to be strongly associated with cigarette smoking.And at the same time,significant positive dose-effect relationship was found both in the daily cigarette consumption and the smoking duration.These findings suggest that the smoking exposure is a specific risk factor for the precancerous gastric lesions.Tobacco contains known gastric carcinogens in experimental animals,which are swallowed directly into the stomach by smokers[18].Smokingrelated DNA adducts have been detected in gastric mucosa of smokers in greater concentrations than in that of nonsmokers[19].In addition,smoking has been reported to increase mRNA expression of certain chemokines in gastric mucosa,which may augment inflammatory reactions[20].On the other hand,reduced gastric defense mechanisms among smokers may contribute to the progression of precursor lesions.Thus,it is biologically plausible that tobacco products play a causal role in gastric carcinogenesis process.
Excessive alcohol consumption causes damage to stomach by impairing the integrity of the mucosal barrier.A recent study from Japan showed that alcohol habits additionally promoted the risk for gastric cancer in subjects with gastric atrophy[8].In our study,we also found an adverse effect of consumption of alcohol on the prevalence of gastric superficial and gastric ulcer.However,contrast to the current study,other studies found that drinking alcohol was either a protective factor or not associated with precancerous gastric lesions such as peptic ulcer[21].Drinking moderate amounts of alcohol has also been shown to protect against active infection with H.pylori[22].The possibility exists that alcohol consumption may be related to positive or negative risk factors,depending on the dose and type of drinking.
In our study the dietary habits were identified as the risk factors for precancerous gastric lesions.Salt is not a directly acting carcinogen,but it is thought to increased the risk of gastric cancer through direct damage to the gastric mucosa,which results in gastritis,increased DNA synthesis,and cell proliferation[23].The relationship between milk intake and gastric diseases including gastric cancer is controversial.In this study,we found no associations with milk.Higher intake of milk or dairy products have often been found among stomach cancer patients than among controls[25]and it has been interpreted that the association is more likely to be a consequence of the disease (i.e.,that patients took these foods to mitigate their symptoms).Although gastric precancerous lesions are generally less symptomatic,Kato et al.found a marginal positive association with dairy consumption[24]and others have reported a significant positive association between milk consumption and the risk of chronic AG[26].Hamajima et al.[27]raised a possibility that milk consumption increased the prevalence of H.pylor infection among individuals with specific genetic background,although no biological mechanisms have been provided.In addition,it is worth noting that H.pylor has been detected in milk from cow[28]and sheep[29],which suggests possible transmission through these products.
Garlic is known to inhibit H.pylori colonization,decrease gastric inflammation by inhibiting cytokine and chemokine release,and repress precancerous changes by inhibiting nuclear factor-kappa B DNA binding,inducing profuse levels of apoptosis and inhibiting mutagenesis[30].After adjusted the effects of the other factors,the results of this study showed that garlic eating may confer protection against the development of gastric ulcer (OR=0.55,95%CI:0.33-0.92).A study that investigated the effect of garlic extract on H.pylori induced gastritis in Mongolian gerbils revealed that gastritis decreased in a dose-dependent manner[31].Eating garlic is traditional in many countries,and several trials have yielded results on its effects on H.pylori infection.The Shandong Intervention Trial reported that garlic supplement interventions could reduce the prevalence of precancerous gastric lesions in Shandong Province in China[32].Some even suggested combined garlic and omeprazole instead of the standard quadruple therapy for the eradication of H.pylori infection[33].However,a randomized trial of the effect of long-term garlic supplementation on the prevalence of precancerous gastric lesions has been reported no statistically significant results for reducing the prevalence of precancerous gastric lesions with garlic supplements[34].Although garlic intake for a long duration did not appear to have an effect on the prevalence of H.pylori infection,garlic-consuming participants had a significantly lower average antibody titer than non-garlic consuming groups.This might suggest an indirect inhibitory effect on the reproduction of H.pylori and possibly the progression to more serious peptic ulcer diseases[35].
In conclusion,our study indicates that dietary habits,smoking and drinking are the risk factors for precancerous gastric lesions.So,interventions against cigarette smoking and bad dietary habits may be important for the prevention of precancerous gastric lesions.As part of the strategy for the cancer control,healthy lifestyle should be emphasized as every one’s responsibility.
[1]Sambasivaiah K,Ibrarullah M,Reddy MK,et al.Clinical profile of carcinoma stomach at a tertiary care hospital in south India[J].Trop Gastroenterol 2004; 25: 21-6.
[2]Samarasam I,Chandran BS,Sitaram V,et al.Palliative gastrectomy in advanced gastric cancer: is it worthwhile[J]? ANZ J Surg 2006; 76: 60-3.
[3]Murray CJ,Lopez AD.Alternate projections of mortality and disability by cause 1990-2020: Global burden of disease study[J].Lancet 1997; 349: 1498-504.
[4]Recalling the Third National Survey on the Cause of Death[M].Chen Zhu,Ed.Beijing: Peking Union Medical College Press 2008.
[5]Correa P.Human gastric carcinogenesis: a multistep and multifactorial process: First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention[J].Cancer Res 1992; 52: 6735-40.
[6]Módena JL,Acrani GO,Micas AF,et al.Correlation between Helicobacter pylori infection,gastric diseases and life habits among patients treated at a university hospital in Southeast Brazil[J].Braz J Infect Dis 2007; 11:89-95.
[7]Kim N,Park YS,Cho SI,et al.Prevalence and risk factors of atrophic gastritis and intestinal metaplasia in a Korean population without significant gastroduodenal disease[J].Helicobacter 2008; 13:245-55.
[8]Yamaji Y,Watabe H,Yoshida H,et al.High-risk population for gastric cancer development based on serum pepsinogen status and lifestyle factors[J].Helicobacter 2009; 14: 81-6.
[9]Yuan Yuan,Zhang Yinchang.A report of prevention and control scene for gastric cancer in Zhuanghe region,Liaoning Province[J].China Tumor 2009; 18:14-8.
[10]You WC,Li JY,Blot WJ,et al.Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer[J].Int J Cancer 1999; 83:615-9.
[11]Correa P.The biological model of gastric carcinogenesis[J].IARC Sci Publ 2004; 157: 301-10.
[12]You WC,Blot WJ,Li JY,Chang YS,Jin ML,Kneller R.Precancerous gastric lesions in a population at high risk of stomach cancer[J].Cancer Res 1993;53:1317-21.
[13]Schubert ML.Gastric secretion[J].Curr Opin Gastroenterol 2008; 24: 659-64.
[14]Anda RF,Williamson DF,Escobedo LG.Selfperceived stress and the risk of peptic ulcer disease: a longitudinal study of US adults[J].Arch Intern Med 1992; 152: 829-33.
[15]La Torre G,Chiaradia G,Gianfagna F,et al.Smoking status and gastric cancer risk: an updated metaanalysis of case-control studies published in the past ten years[J].Tumori 2009; 95:13-22.
[16]Shikata K,Doi Y,Yonemoto K,et al.Populationbased prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: the Hisayama Study[J].Am J Epidemiol 2008; 168:1409-15.
[17]Koivisto TT,Voutilainen ME,Färkkilä MA.Effect of smoking on gastric histology in Helicobacter pyloripositive gastritis[J].Scand J Gastroenterol 2008;43:1177-83.
[18]Saha SK.Smoking habits and carcinoma of the stomach: a case-control study[J].Jpn J Cancer Res 1991; 82: 497-502.
[19]Dyke GW,Craven JL,Hall R,et al.Smoking-related DNA adducts in human gastric cancers[J].Int J Cancer 1992; 52: 847-50.
[20]Figueroa JD,Terry MB,Gammon MD,et al.Cigarette smoking,body mass index,gastroesophageal reflux disease,and non-steroidal antiinflammatory drug use and risk of subtypes of esophageal and gastric cancers by P53 overexpression[J].Cancer Causes Control 2009; 20:361-8.
[21]Módena JL,Acrani GO,Micas AF,et al.Correlation between Helicobacter pylori infection,gastric diseases and life habits among patients treated at a university hospital in Southeast Brazil[J].Braz J Infect Dis 2007; 11: 89-95.
[22]Murray LJ,Lane AJ,Harvey IM.Inverse relationship between alcohol consumption and active Helicobacter pylori infection: the Bristol Helicobacter project[J].Am J Gastroenterol 2002; 97: 2750-5.
[23]Leung WK,Wu KC,Wong CY,et al.Transgenic cyclooxygenase-2 expression and high salt enhanced susceptibility to chemical-induced gastric cancer development in mice[J].Carcinogenesis 2008; 29:1648-54.
[24]Kato I,Vivas J,Plummer M,et al.Environmental factors in Helicobacter pylori-related gastric precancerous lesions in Venezuela[J].Cancer Epidemiol Biomarkers Prev 2004; 13: 468-76.
[25]Munoz N,Plummer M,Viva J.A case-control study of gastric cancer in Venezuela[J].Int J Cancer 2001;93: 417-23.
[26]Fontham E,Zavala D,Correa P.Diet and chronic atrophic gastritis: a case-control study[J].J Natl Cancer Inst 1986; 76: 621-7.
[27]Hamajima N,Matsuo K,Suzuki T.Low expression myeloperoxidase genotype negatively associated with Helicobacter pylori infection[J].Jpn J Cancer Res 2001; 92: 488-93.
[28]Fujimura S,Kawamura T,Kato S,et al.Detection of Helicobacter pylori in cow’s milk[J].Lett Appl Microbiol 2002; 35: 504-7.
[29]Dore MP,Sepulveda AR,El-Zimaity H.Isolation of Helicobacter pylori from sheep—implications for transmission to humans[J].Am J Gastroenterol 2001;96:1396-401.
[30]Lee SY,Shin YW,Hahm KB.Phytoceuticals: mighty but ignored weapons against Helicobacter pylori infection[J].J Dig Dis 2008; 9:129-39.
[31]Iimuro M,Shibata H,Kawamori T.Suppressive effects of garlic extract on Helicobacter pyloriinduced gastritis in Mongolian gerbils[J].Cancer Lett 2002; 187: 61-8.
[32]Gail MH,Pfeiffer RM,Brown LM.Garlic,vitamin,and antibiotic treatment for Helicobacter pylori: a randomized factorial controlled trial[J].Helicobacter 2007; 12: 575-8.
[33]Fani A,Fani I,Delavar M.Combined garlicomeprazole versus standard quadruple therapy for eradication of Helicobacter pylori infection[J].Indian J Gastroenterol 2007; 26: 145-6.
[34]You WC,Brown LM,Zhang L,et al.Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions[J].Natl Cancer Inst 2006; 98: 974-83.
[35]Salih BA,Abasiyanik FM.Does regular garlic intake affect the prevalence of Helicobacter pylori in asymptomatic subjects[J]? Saudi Med J 2003; 24:842-5.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Methylation and Demethylation of Ink4 Locus in Cancer Development
- Apoptotic B16-F1 Cells Coated with Recombinant Calreticulin Mediated Anti-tumor Immune Response in Mice
- Polymorphisms of UGT1A7 and XRCC1 are Associated with an Increased Risk of Hepatocellular Carcinoma in Northeast China
- Stanniocalcin-1 Detection of Peripheral Blood in Patients with Colorectal Cancer
- Promoter Hypermethylation of KiSS-1 Gene in Gastric Cancer
- Recurrent Patterns and Factors Involved in Node-negative Advanced Gastric Cancer
