Apoptotic B16-F1 Cells Coated with Recombinant Calreticulin Mediated Anti-tumor Immune Response in Mice
2010-01-08ChunyuCaoYuHanYushanRenYanlinWang
Chun-yu Cao,Yu Han,Yu-shan Ren,Yan-lin Wang
Three Gorges University Medical College,Three Gorges University Institute of Molecular Biology,Yichang 443002,China
INTRODUCTION
Malignant cells are usually very low in antigenicity with decreased or no expression of immune recognition signals,that makes mutated cells escape from the clearance by immune system[1-4].In order to elicit immune system to effectively clear mutated cells,researchers have been working to find a way to recover or stimulate body’s specific antitumor immune response in vivo.
Calreticulin (CRT)is a highly conservative Ca2+-binding protein that is ubiquitous in mammalian karyocytes and mostly present in endoplasmic reticulum (ER)lumen.CRT has multiple biological functions which are relevant to its subcellular localization[5-11].Recently,researchers discovered that CRT located on the membrane of apoptotic cells was the key molecule involved in the recognition and clearance of apoptotic cells[12-15].It has been reported that when mouse CT-26 colon carcinoma cells were treated by antitumor chemical anthracyclin,along with the cell apoptosis,the CRT was quickly transferred from ER onto the membrane surface[16].Importantly,these CRT-coated apoptotic tumor cells could be used as cell-antigen to elicit specific anti-tumor immune response in tumor bearing mice[17-18].This kind of effect was not observed when apoptotic tumor cells without CRT coating on the surface were used as the antigen.These researches suggested that the CRT on the surface of apoptotic tumor cells has a potential value for tumor immunotherapy.Since CRT translocation from ER to the cell surface is not an inherent feature for apoptosis in tumor cells,but only events induced by specific stimulus in some tumor cell lines,a method that can easily coat CRT onto the surface of any tumor cells would very helpful to provide a potent research too l for anti-tumor immunotherapy[19].In this study,we used recombinant CRT to artificially coat apoptotic mouse melanoma B16-F1 tumor cells and then used them as cell-antigen to study the ability of these artificially CRT-coated B16-F1 cells in induction of specific antitumor immune responses.
MATERIALS AND METHODS
Cell lines and Experimental Animals
The mouse melanoma cell line B16-F1 and hepatocellular carcinoma cell line H22 were maintained in our laboratory.BALB/c SPF mice (7-8 weeks old,35 male,35 female),were supplied by Hubei Experimental Animal Center (No.0001839).All the animals were housed under specific pathogen free conditions and the animal experiments were conducted according to the guidelines of Animal Experimentation Ethics Committee,The Chinese Three Gorges University.
Drugs and Chemicals
Trizol RNA purification reagent,RPMI-1640,penicillin,streptomycin,3-(4,5- Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT),phenazine methosulfate and nitrotetrazoliumbluechlorid were purchased from Sigma Co.(St.Louis,MO,USA).Bis-ethyl-norspermine (BENS)was kindly provided by Prof.Robert A.Casero (Johns Hopkins University,USA).SYBR PrimeScript Kit was obtained from TaKaRa Co.(Otsu,Japan).Lymphocyte separation medium was from DaKewe Biotech Co.(Shengzhen,China).Rabbit anti-human recombinant CRT pAb and rabbit anti-mouse actin mAb were obtained from Stressgen Co.(Ann Arbor,MI,USA).Goat anti-rabbit IgG-HRP and goat anti-rabbit IgG-Rhodamine123 were from Zhongshan Goldenbrige Biotechnology Co.(Beijing,China).PVDF membrane was obtained from Bio-Rad(Hercules,CA,USA).ECL detection system was a product of Amersham Pharmacia Biotech (Uppsala,Sweden).All primer synthesis was performed by Sangon Biological Engineering Technology &Services Co.(Shanghai,China).
Cell Culture
Cells were cultured in RPMI-1640 medium supplemented with 10% (v/v)calf serum,100μg/ml streptomycin and 100units/ml penicillin in a humidified 5% CO2incubator at 37°C.
Cellular Proliferation Assay
B16-F1 cells were seeded into 96-well culture plates (4,000 cells/well in 50μl standard RPMI-1640 medium)in triplicate.Twenty four hours later,the cells were treated by BENS at different concentrations ranging from 1 to 20μmol/L in 50μl medium.The cells treated with equal amount of normal medium instead of drug were served as control.After drug treatment for 24 h and 48 h,the medium in each well was removed and 200μl MTT solution (0.25 g/L in RPMI-1640 medium)was added.After 4 h incubation at 37°C,the medium was removed and 200μl dimethyl sulfoxide was added into each well.After 30 min incubation at room temperature,absorbance (A)at 570nm was recorded.The cell survival rate was calculated as follows: Cell survival rate = Adrug/Acontrol × 100%.
Detection of Apoptosis by DNA Fragmentation Electrophoretic Analysis
B16-F1 cells were treated with 10 μmol/L BENS for 48 hours and harvested (the suspended apoptotic cells in medium were also harvested)by centrifuging at 2 000 r/min for 5 minutes.The cells were then lysed in 0.3 mL cell lysis buffer (50 mmol/L Tris-HCl,20 mmol/L EDTA,0.5% Triton X-100,pH 8.0)for 20minutes on ice.Nuclei were removed by centrifuging at 12 000 r/min for 10 min and then the supernatant were obtained and extracted with phenol/chloroform.The DNA fragments in extracted supernatants were precipitated in 2 volumes of 100%ethanol and 1/10 volume of 3 mol/L sodium acetate(pH 5.2),and the pellets were finally resuspended in 0.1×SSC buffer.After treatment with DNase-free RNase A (50 mg/mL)for 30 minutes at 37°C,NaCl was added to 1 mol/L final concentration and the solution was extracted again with phenol/chloroform and precipitated by ethanol.The pellets were suspended in 30μl ddH2O and loaded into a 2% (w/v)agarose gel for electrophoresis.
Quantitative Real-time PCR
The CRT mRNA levels in B16-F1 cells were detected by quantitative real-time PCR (QT-RT-PCR)and expressed relatively to the expression of -actin.The first-strand cDNA was synthesized from total RNA of B16-F1 cells by reverse transcription.Real-time PCR was performed with SYBR PrimeScriptTM Kit using Opticn2 Thermal Cycler(MJ Research Inc.,Walthan,MA,USA).Primers used for amplifying CRT were 5’-GATGGATGGAGAGTG GGAACC-3’/5’-GAGATCTAGGCCCAGTACAGC-3’ and primers for β-actin were 5’-TGGCACCCAGC ACAATGAA-3’/5’-CTAAGTCATAGTCCGCCTAA-3’.The PCR cycling condition was as follows: 95°C 5 min; 94°C 30 s,60°C 30 s,72°C 30 s,78°C 1 s for plate reading,30 cycles; 72°C 10 min.The quantitative analysis of the data was performed using Opticon Monitor 2.02.24 software.The relative difference of gene expression was calculated as follows:
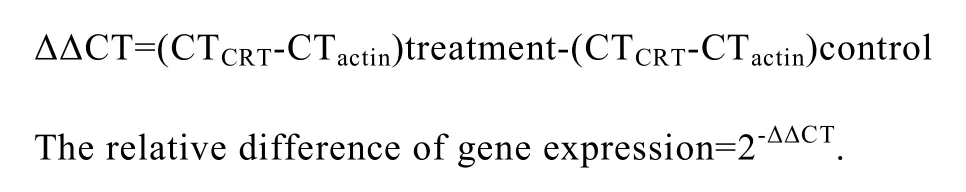
Western Blot Analysis
B16-F1 cells were cultured in RPMI-1640 medium containing BENS (10μmol/L)for 48 hours and harvested.The cells then were treated with cell lysis buffer (50mmol/L Tris-HCl,20mmol/L EDTA,0.5% Triton X-100,pH 8.0)for 20 min on ice.Nuclei and cytoplasm were separated from each other by centrifugation at 1000 r/min for 5 minutes.Nuclei were washed once with 1mL ice-cold PBS.A total of 5µg of nuclei or cytoplasm proteins from each sample was denatured by boiling,resolved by 10% SDSPAGE and transferred onto PVDF membranes.After blocking with 5% (w/v)non-fat milk,the blots were probed with specific primary Abs and visualized with the appropriate HRP-conjugated secondary Abs and an ECL detection system.β-actin was used as a loading control in this study.
Immunofluorescence Detection of Calreticulin in B16-F1
Immunofluorescence was used to determine the subcellular localization of CRT.B16-F1 cells were seeded into 12-well culture plates (2×104/well in 1 ml standard RPMI-1640 medium).Twenty four hours later,the cells were treated with BENS (10μmol/L)in 1mL medium for 48 hours.B16-F1 cells treated with equal amount of normal medium instead of drugs were served as the control group.The medium was discarded and the cells were washed with PBS and treated with 4% (w/v)paraformaldehyde for fixation.After fixation,the cells were washed with ice-cold PBS,incubated with 10% (v/v)goat serum for blocking and reacted with anti-CRT pAbs over night at 4°C.The cells were then washed with ice-cold PBS,incubated with Rhodamine-123 labeled anti-rabbit IgG for 45 minutes at 37.℃After that,the cells were stained with 300nmol/L DAPI (4′,6′-diamidino-2-phenylindole)for nuclei,washed with PBS,enclosed in 50% glycerol (diluted by 0.01 mol/L PBS,pH 8.0)and examined by fluorescence/phase contrast microscopy (TE2000S,Nikon,Japan).
The Expression and Purification of Recombinant CRT
Recombinant CRT (rCRT)was prepared as reported before[17].Simply,the coding sequence for mouse CRT was acquired from the total RNA of mouse liver by reverse transcription PCR,cloned into pET-15b vector and then transformed into E.coli.Rossetta(DE3).After IPTG induction,rCRT expressed in the host cells was purified by Ni-NTA affinity chromatography in denature condition and then dialyzed to recovery its natural property.
Animal Experiments
BALB/c SPF mice (35 male,35 female,n=70)were randomly divided into 4 groups: 1)PBS group(blank control,n=14),2)B16 group (tumor positive control,n=20),3)B16-BENS group (n=18),4)B16-BENS-rCRT group(n=18).For immuneinoculation,B16-F1 cells were treated by 10 mol/L BENS for 48h to induce apoptosis and harvested by 2 mmol/L EDTA digestion.The cells were re-suspended in PBS,adjusted to 5×106/mL and subcutaneously inoculated into the back of each mouse (100μL for each)in the B16-BENS group.To coating apoptotic B16-F1 cells with rCRT,apoptotic B16-F1 cells induced by BENS as described above were incubated with 30μg/mL rCRT in RPMI-1640 medium (1% calf serum)for 30 minutes at 37°C and washed with ice-cold PBS to remove free rCRT.These rCRT coated B16-F1 cells were then subcutaneouly inoculated into the back of each mouse in B16- BENS-rCRT group in the same cell number as B16- BENS group.PBS instead of B16-F1 cells was used to inoculate other two control groups of mice at the same time.The secondary animal immunization was performed 10 days after the first immunization.Ten days after the second immunization,living B16-F1 cells (5.0×105cells in 100μl PBS for each)were subcutaneouly injected into the back of each mouse in B16 group,B16-BENS group and B16-BENS-rCRT group respectively.The mice in PBS group were injected with PBS as the tumor-free control.Then mice were monitored once every two days after injection.Appearance of black-blue spots in inoculated area was regarded as the beginning of the tumor growth.
Lactate Dehydrogenase (LDH)Release Assay
Splenocytes were obtained from the experimental mice and isolated with lymphocyte separation medium by centrifugation at 2 500 r/min for 10 min at 4°C and then used as effector cells in the LDH release assay.These splenocytes were seeded into 96-well culture plates (1×106cells/well)in 100μl RPMI-1640 medium without phenolsulphonphthalein.B16-F1 cells (target cells)were then seeded into the wells that contained splenocytes in 100μl in RPMI-1640 medium in three different target/effector cells ratios (1:25,1:50,1:100).In the natural release group,target cells were replaced by equal volume of medium.In the maximum release group,the effector cells were replaced by equal volume of 0.1% NP-40.After incubation for 3 h,the cell culture plates were centrifuged at 2000 r/min for 5 min.Cell medium were then transferred to fresh culture plates(100μl/well)and equal volume of LDH substrate reaction buffer (80 μg/mL Phenazine Methosulfate,320μg/mL Nitrotetrazoliumbluechlorid,800μg/mL codehydrogenase I,sodium lactate 40mmol/L)were added.After 10-min incubation at 37°C in dark,enzyme reactions were stopped by citric acid(30μl/well)and then absorbance (A)at 450 nm was recorded at room temperature.The rate of cytolysis was calculated as follow: Rate of cytolysis = (Aeffector
Statistical Analysis
RESULTS
BENS Inhibited Growth of B16-F1 Cells by Inducing Apoptosis
BENS,a polyamine analogue,has been proved to be an effective anti-tumor agent[18].In this study,the sensitivity of B16-F1 cells to BENS was determined by MTT cellular survival assay after the cells were treated by BENS at concentrations ranging from 1 to 20μmol/L for 24h or 48h.The results showed that B16-F1 cells exhibited a dose-dependent growth inhibition after exposure to BENS treatment(Table 1).
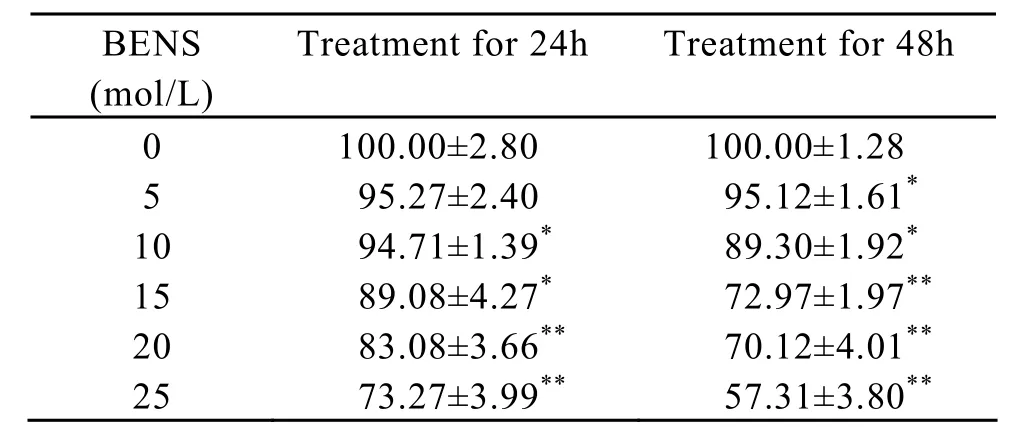
Table 1.BENS inhibits the growth of B16-F1 cells
DNA fragments in the cytoplasm of B16-F1 cells were isolated after 48 hours treatment with BENS (10 μmol/L)and then separated by agarose gel electrophoresis.The typical apoptotic DNA ladder was observed in the samples from the BENS-treated cells.The results showed that BENS effectively induced apoptosis of B16-F1 cells (Figure 1).
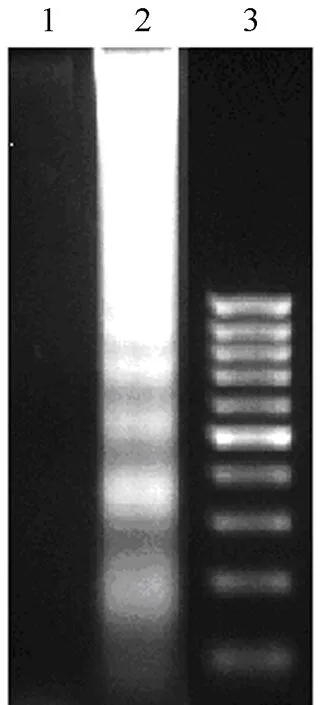
Figure 1.BENS induced apoptosis of B16-F1 cells as assayed by DNA fragmentation.
Expression and Subcellular Localization of CRT in BENS-treated B16-F1 Cells
To evaluate the effects of BENS on CRT expression and subcellular localization,CRT was determined by Western blot assay.No obvious difference of CRT contents was observed between BENS-treated and control B16-F1 cells in both nuclei and cytoplasm (Figure 2A).The mRNA levels of CRT in BENS-treated and control cells were also detected by quantitative real-time PCR.Again there was no significant difference of CRT mRNA between BENS-treated and control B16-F1 cells (Figure 2B).
The subcellular localization of CRT in cells was determined by immunofluorescence detection.The results showed that no obvious membrane translocation and CRT-coating was observed in B16-F1 cells after BENS treatment (Figure 3).The data mentioned above proved that BENS could induce apoptosis of B16-F1 cells but did not induce redistribution of CRT within the cells.
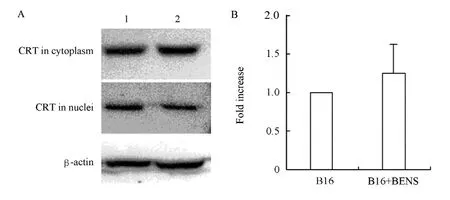
Figure 2.The effect of BENS treatment on CRT expression in B16-F1 cells.A: Effect of BENS treatment on CRT expression at protein level,Line 1: B16-F1 control cells; Line 2: B16-F1 cells treated by 10 μmol/L BENS for 48 h; B: Effect of BENS treatment on CRT expression at mRNA level.Each bar presents the±s (n=3).Data was assessed by Student’s two-tailed t-test.
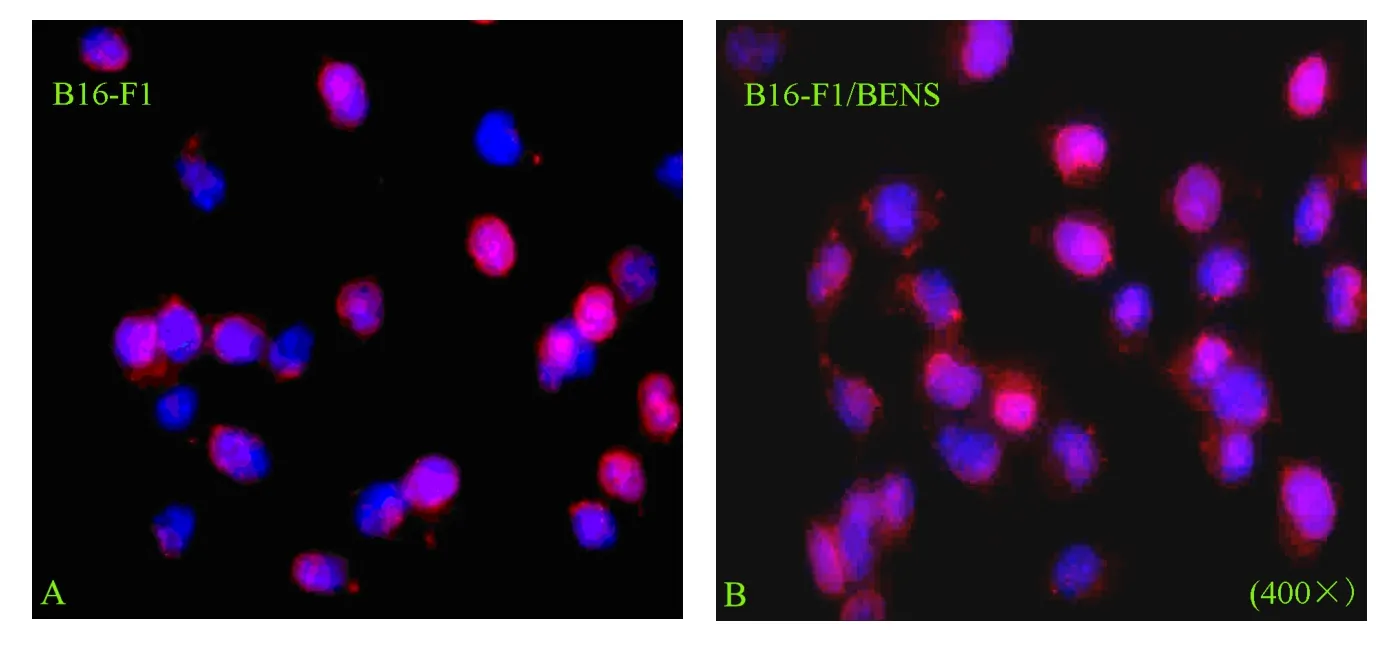
Figure 3.The effect of BENS treatment on the distribution of CRT in B16-F1 cells.The red color represented CRT recognized by rabbit anti-CRT antibody and Rhodamine-123 labeled anti-rabbit IgG.Blue color indicates the nuclei stained with DAPI.A:B16-F1 control cells.B: B16-F1 cells treated by 10μmol/L BENS for 48 h.
rCRT-coated B16-F1 Cells Induced An Specific Anti-tumor Effect against Homogeneous Tumor Cells in Mice
To evaluate the ability of rCRT-coated B16-F1 cells in inducing specific anti-tumor effect against homogeneous tumor cells in mice,rCRT-coated apoptotic B16-F1 cells induced by BENS were used as the cell-antigen to immunize mice.The results showed that the specific antitumor effect was clearly induced in the immunized animals (Figure 4).Ten days after rechallenge by live B16-F1 cells,a much lower percentage of tumor generation (44.4%)in the mice of B16-BENS-rCRT group was observed,compared with a higher percentage in the mice of B16-BENS group (67%)or B16 group (85%).The results demonstrated that rCRT-coating enhances antigenicity of apoptotic B16-F1 cells and stimulates an effective immune response against homogeneous tumor cells in mice.

Figure 4.The inoculation of rCRT-coated apoptotic B16-F1 cells decreased the percent of tumor generation in BALB/c mice rechallenged by live B16-F1 cells.Data of all these groups was assessed by Fisher’s exact probabilities in 2×2 table.*P<0.05 vs B16 positive control mice.
Immunizing Mice with rCRT-coated Apoptotic B16-F1 Cells Increased Cytolytic Effect of Splenocyts
In order to prove the effect of immune-activation stimulated by rCRT-coated B16-F1 cells,the specific cytolytic effect of the splenocytes from experimental animals on B16-F1 cells was determined by LDH release assay in which the splenocytes from 4 groups of mice were used as effectors and B16-F1 cells were used as targets.In a parallel assay,H22 cells,as a non-homologous control tumor cells,were used as target cells to prove the specificity of cytolysis.The results showed that,in contrast to control group,splenocytes from mice in B16-BENS-rCRT group exhibited a significant higher cytolytic efficiency against B16-F1 cells than other mice (Figure 5A).In the parallel experiment,splenocytes from all groups of mice exhibited a similar cytolytic efficiency against H22 cells (Figure 5B).The results suggested that the immune response induced by rCRT-coated apoptotic cells was specific against homologous tumor cells.
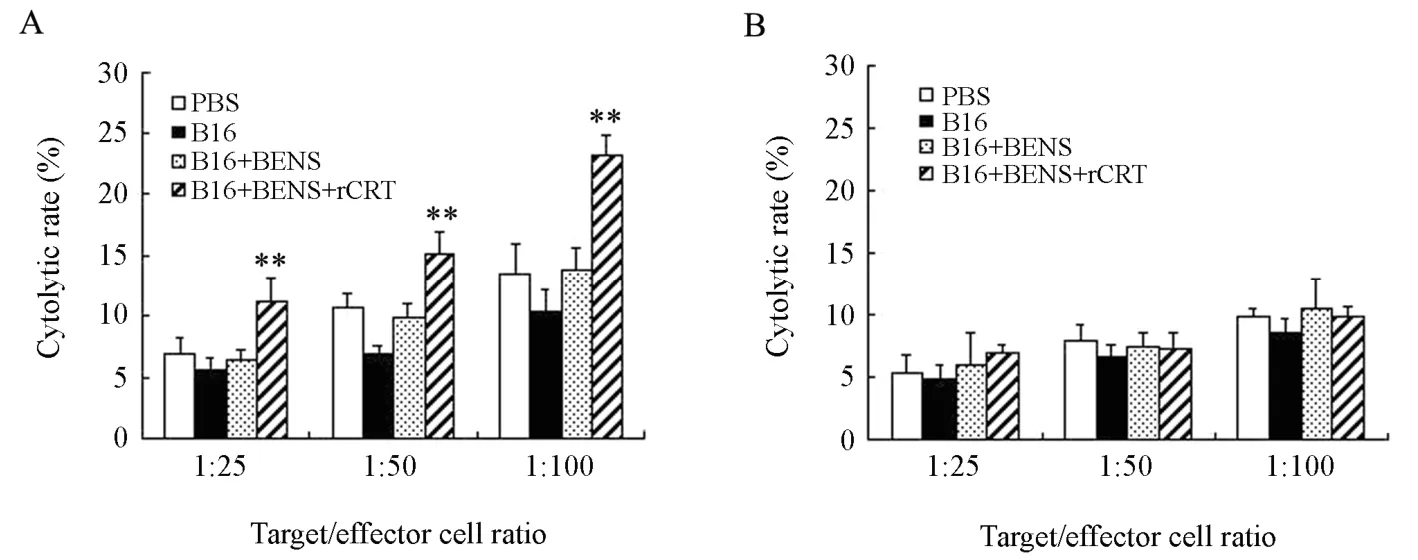
Figure 5.Enhanced cytolytic effect of splenocytes on homogeneous tumor cells in mice inoculated with rCRT coated apoptotic cells.A: B16-F1 cells were used as the target cells.B: H22 cells were used as target cells.Each bar presents the ±s (n=3),Data was assessed by Student’s two-tailed t-test.**P<0.01vs PBS negative control mice.
DISCUSSION
One of the key steps of this study is to obtain CRT-coated apoptotic B16-F1 tumor cells,thus the selection of apoptosis inductor is important.The drug should induce apoptosis in B16-F1 cells but not the subcellular localization of CRT.Otherwise,it will be difficult to tell whither the membrane CRT comes from the artificial coating.Polyamine analogues,a class of chemical agent synthesized artificially,have the similar chemical structure to the natural polyamines and potent effect against tumor proliferation[20-22].In this study,we discovered that BENS,a member of polyamine analogue family,could induce apoptosis in B16-F1 cells,without obvious interference on the subcellular localization and gene expression of CRT,meeting our requirement for drug selection.
After B16-F1 cells were treated by BENS to induce apoptosis and incubated with rCRT to coat rCRT onto the membrane,the cells were used as cell-antigen to immunize BALB/c mice.The results showed that these rCRT-coated B16-F1 cells elicited an inhibitive effect against the homologous tumor cells.Comparing with the positive control group,the percentage of melanoma generation decreased obviously in mice immunized with rCRT-coated apoptotic B16-F1 cells (B16-BNES-rCRT group)when mice were re-challenged with live B16-F1 cells.But no statistical difference existed between the positive control group and B16-BENS group without coating of rCRT.The results proved that the artificially coated CRT on the surface of apoptotic tumor cells mediated an important anti-tumor immune response.LDH release assay showed that only splenocytes from mice in B16-BNES-rCRT group obviously increased the cytolytic effect against B16-F1 cells and no such effect was discovered when non-homologous H22 cells were used as target cells.
These results demonstrated that rCRT-coated apoptotic cells elicited specific immune response against homologous tumor cells in mice.The possible mechanism is that the rCRT coated on the tumor cell membrane can be used as an identification signal that would promote recognition and phagocytosis of tumor cells by immune cells.The tumor antigens are then processed within the immune cells and presented to stimulate effective anti-tumor immune response against homologous tumor cells in vivo[23].
These experimental results suggest that rCRT coated on the cell surface can enhance immunogenicity of apoptotic tumor cells and mediated effective anti-tumor immunoresponse in mice.
Acknowledgements
The authors thank Dr.Robert A.Casero in Johns Hopkins University for his support in this study.
[1]Rescigno M,Avogadri F,Curigliano G.Challenges and prospects of immunotherapy as cancer treatment[J].Biochim Biophys Acta 2007; 1776: 108-23.
[2]Prestwich RJ,Errington F,Hatfield P,et al.The Immune system,is it relevant to cancer development,progression and treatment[J]? Clin Oncol(R Coll Radiol)2008; 20: 101-12.
[3]Reiman JM,Kmieciak M,Manjili MH,et al.Tumor immunoediting and immuno-sculpting pathways to cancer progression[J].Semin Cancer Biol 2007; 17:275-87.
[4]Smyth MJ.Tumour immunology[J].Curr Opin Immunol 2007; 19: 200-2.
[5]Fliegel L,Burns K,MacLennan DH,et al.Molecular cloning of the high affinity calcium-binding protein(calreticulin)of skeletal muscle sarcoplasmic reticulum[J].J Biol Chem 1989; 264: 21522-8.
[6]Burns K,Duggan B,Atkinson EA,et al.Modulation of gene expression by calreti-culin binding to the glucocorticoid receptor[J].Nature 1994; 367: 476-80.
[7]Mery L,Mesaeli N,Michalak M,et al.Overexpression of calreticulin increases intracellular Ca2+storage and decreases store-operated Ca2+influx[J].J Biol Chem 1996; 271: 9332-9.
[8]Lewis JW and Elliott T.Evidence for successive peptide binding and quality control stages during MHC class I assembly[J].Curr Biol 1998; 8: 717-20.
[9]Arosa FA,deJesus O,Porto G,et al.Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex I molecules[J].J Biol Chem 1999; 274:16917-22.
[10]Fraser SA,Karimi R,Michalak M,et al.Perforin lytic activity is controlled by calreticulin[J].J Immunol 2000; 164: 4150-5.
[11]Qiu Y,Michalak M.Transcriptional control of the calreticulin gene in health and disease[J].Int J Biochem Cell Biol 2009; 41: 531-8.
[12]Henson PM,Bratton DL,Fadok VA.Apoptotic cell removal[J].Curr Biol 2001; 11: R795-805.
[13]Hoffmann PR,deCathelineau AM,Ogden CA,et al.Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells[J].J Cell Biol 2001; 155:649-59.
[14]Ogden CA,deCathelineau A,Hoffmann PR,et al.C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells[J].J Exp Med 2001; 194: 781-95.
[15]Tarr JM,Young PJ,Eggleton P,et al.A Mechanism of Release of Calreticulin from Cells During Apoptosis[J].J Mol Biol 2010; 401: 799-812.
[16]Gardai SJ,McPhillips KA,Frasch SC,et al.Cellsurface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte[J].Cell 2005; 123: 321-34.
[17]Obeid M,Tesniere A,Ghiringhelli F,et al.Calreticulin exposure dictates the immunoenicity of cancer cell death[J].Nat Med 2007; 13: 54-61.
[18]Panaretakis T,Kepp O,Kroemer G,et al.Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death[J].EMBO J 2009; 28: 578-90.
[19]Cao Y,Han Y,Wang Y,et al.Mitoxantrone-Mediated Apoptotic B16-F1 Cells Induce Specific Anti-tumor Immune Response[J].Cell Mol Immuno 2009; 6:469-75.
[20]Huang Y,Pledgie A,Casero RA Jr,et al.Molecular mechanisms of polyamine analogs in cancer cells[J].Anticancer Drugs 2005; 16: 229-41.
[21]Casero RA,Marton LJ.Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases[J].Nat Rev Drug Discov 2007; 6: 373-90.
[22]Casero RA Jr,Frydman B,Stewart TM,et al.Significance of targeting polyamine metabolism as an antineoplastic strategy: unique targets for polyamine analogues[J].Proc West Pharmacol Soc 2005; 48:24-30.
[23]Zitvogel L,Kepp O,Senovilla L,et al.Immunogenic Tumor Cell Death for Optimal Anticancer Therapy:The Calreticulin Exposure Pathway[J].Clin Cancer Res 2010; 16: 3100-4.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Methylation and Demethylation of Ink4 Locus in Cancer Development
- Polymorphisms of UGT1A7 and XRCC1 are Associated with an Increased Risk of Hepatocellular Carcinoma in Northeast China
- Risk Factors of Precancerous Gastric Lesions in A Population at High Risk of Gastric Cancer
- Stanniocalcin-1 Detection of Peripheral Blood in Patients with Colorectal Cancer
- Promoter Hypermethylation of KiSS-1 Gene in Gastric Cancer
- Recurrent Patterns and Factors Involved in Node-negative Advanced Gastric Cancer
