Bone morphogenetic protein 4 induces cholinergic differentiation of brain-derived neural stem cells in vivo and in vitro★
2010-01-04YanChangJingkunPanLeiTianShuilongGuoYunLuoXinCuiYilongXue
Yan Chang, Jingkun Pan, Lei Tian, Shuilong Guo, Yun Luo, Xin Cui, Yilong Xue
Laboratory of Cell Biology, Institute of Geriatrics, General Hospital of Chinese PLA, Beijing 100853, China
Bone morphogenetic protein 4 induces cholinergic differentiation of brain-derived neural stem cellsin vivoandin vitro★
Yan Chang, Jingkun Pan, Lei Tian, Shuilong Guo, Yun Luo, Xin Cui, Yilong Xue
Laboratory of Cell Biology, Institute of Geriatrics, General Hospital of Chinese PLA, Beijing 100853, China
In vitrostudies have demonstrated that many factors of bone morphogenetic proteins (BMPs) induce cholinergic differentiation of neural stem cells. However, BMP retains the potential to induce increased numbers of cholinergic neurons in central nervous system regions that are rich in cholinergic cells, which is an important determinant of BMP. Therefore, BMP-4 was added to neural stem cell culture medium or the adult rat hippocampal dentate gyrus. Results demonstrated that BMP-4 induced cholinergic differentiation of neural stem cellsin vitroand increased the number of cholinergic neurons in the adult rat hippocampal dentate gyrus.
bone morphogenetic protein; neural stem cell; induced differentiation; cholinergic neuron; neural regeneration
INTRODUCTION
Studies have demonstrated that the majority of non-induced neural stem cells (NSCs) differentiate into GABAergic neurons, and spontaneous differentiation into neurons of other phenotypes is rare[1-4]. Cholinergic neurons participate in learning and memory functions, and the factors that induce cholinergic differentiation of NSCs remain unclear. Evidence suggests that factors of bone morphogenetic proteins (BMPs) are associated with cholinergic differentiation in the central nervous system[5-7]. BMP-9 has been shown to induce differentiation of cultured NSCs into cholinergic neurons[8], and intraventricular BMP-9 injection increases acetylcholine levelsin vivo[9]. In addition, BMP-4 and BMP-2 induce cholinergic differentiation of NSCsin vitro[10-12]. Whether BMPs retain similar effects in the central nervous system remains poorly understood. Therefore, the present study treated NSC culture medium or the adult rat hippocampal dentate gyrus with BMP-4 to determine the role that BMP-4 plays in inducing cholinergic differentiation of brain-derived NSCsin vivoandin vitro.
RESULTS
In vitro experiments Morphology of rat hippocampal and striatal NSCs cultured in vitro
Cells from the hippocampus, striatum, third ventricle, lateral ventricle bands, and subventricular bands were isolated from 2-month-old rats and were observed under an inverted optical microscope immediately following inoculation. The cells were suspended in buffer and exhibited a rounded appearance, with a diameter of 20-30 nm. There were many pyknotic black granules in the cytoplasm of some cells. A piece of flagellum-like appendage was connected to the edge of cells, and the cells spun at various speeds (Figure 1A). After 8 hours in culture, some cells adhered to the culture plate, exhibited a spherical morphology, and were surrounded by strong halation (Figure 1B). After 24 hours, the cells divided, proliferated, and formed cell masses (Figure 1C). After 7 days, some short and thick processes erupted from the soma in the outer region of cell masses; cells left the cell masses and developed into precursor-like cells (Figure 1D). Nestin immunocytochemistry showed that approximately 48% of the cells expressed nestin in the cytoplasm (Figure 2). These findings suggested that cells isolated from the 2-month-old rat hippocampus, striatum, and other regions were NSCs.
BMP-4 induces cholinergic differentiation of rat brain-derived NSCs
Cell morphology: After 24 hours in culture, the brain-derived NSCs were further cultured for 7 days with medium containing BMP-4 (BMP-4 group). After 7 days, approximately 34% of cells exhibited neuronal characteristics,i.e., pyknoticgranules in the cytoplasm, large and round nuclei with a clear nucleolus, each cell had one or several processes, and cells were interconnected by processes (Figure 3A). In comparison, 25% of cells not treated with BMP-4 (control group) exhibited neuronal characteristics.
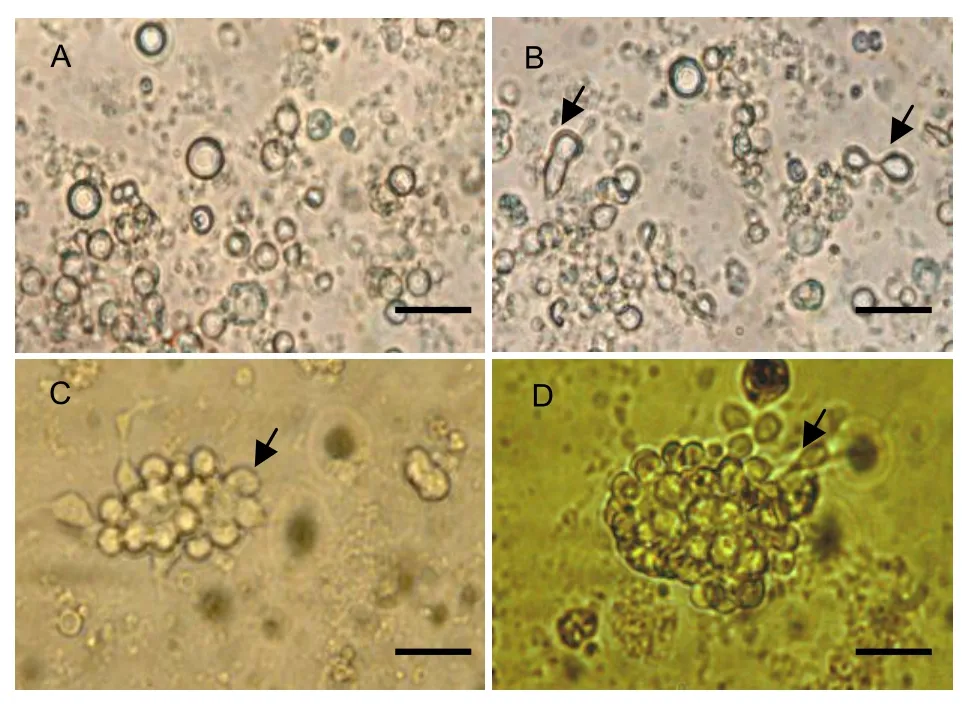
Figure 1 Morphology of neural stem cells isolated from the adult rat hippocampus, striatum, third ventricle, lateral ventricle bands and subventricular bands (inverted optical microscope; scale bars: 20 μm). (A) Immediately after inoculation, cells are suspended in the buffer with a round appearance. (B) At 24 h, the cells divide and exhibit a dumbbell-like shape (arrows). (C) At 2 d, cell masses are observed, composed of several dividing round cells (arrow). (D) At 7 d, larger cell masses form; some cells in the peripheral region sprout short and thick processes, exit the cell masses, and differentiate into precursor-like cells (arrow).

Figure 2 Identification of cells isolated from the adult rat hippocampus, striatum, third ventricle, lateral ventricle bands, and subventricular bands by immunocytochemistry (scale bars: 10 μm). (A) Nestin-positive cells exhibit a round appearance with cytoplasmic expression. (B) Glial fibrillary acidic protein (GFAP)-negative cells. (C) Neurofilament 160-positive cells. (D) GFAP-positive cells.
Double-labeling immunocytochemistry: To determine the effects of BMP-4 induction on cholinergic differentiation of rat brain-derived NSCs, after 7 days in culture, the BMP-4 and control groups were stained with anti-nestin and -choline acetyltransferase (ChAT) antibodies (Figure 3). In the BMP-4 group, approximately 28% of cells exhibited ChAT immunoreactivity, and approximately 38% of cells exhibited nestin immunoreactivity (Figures 3C, D, E). In the control group, only 8% of cells expressed ChAT and approximately 52% of cells expressed nestin. These findings suggested that BMP-4 addition to the culture medium significantly enhanced the proportion of rat brain-derived NSCs differentiating into cholinergic cells.
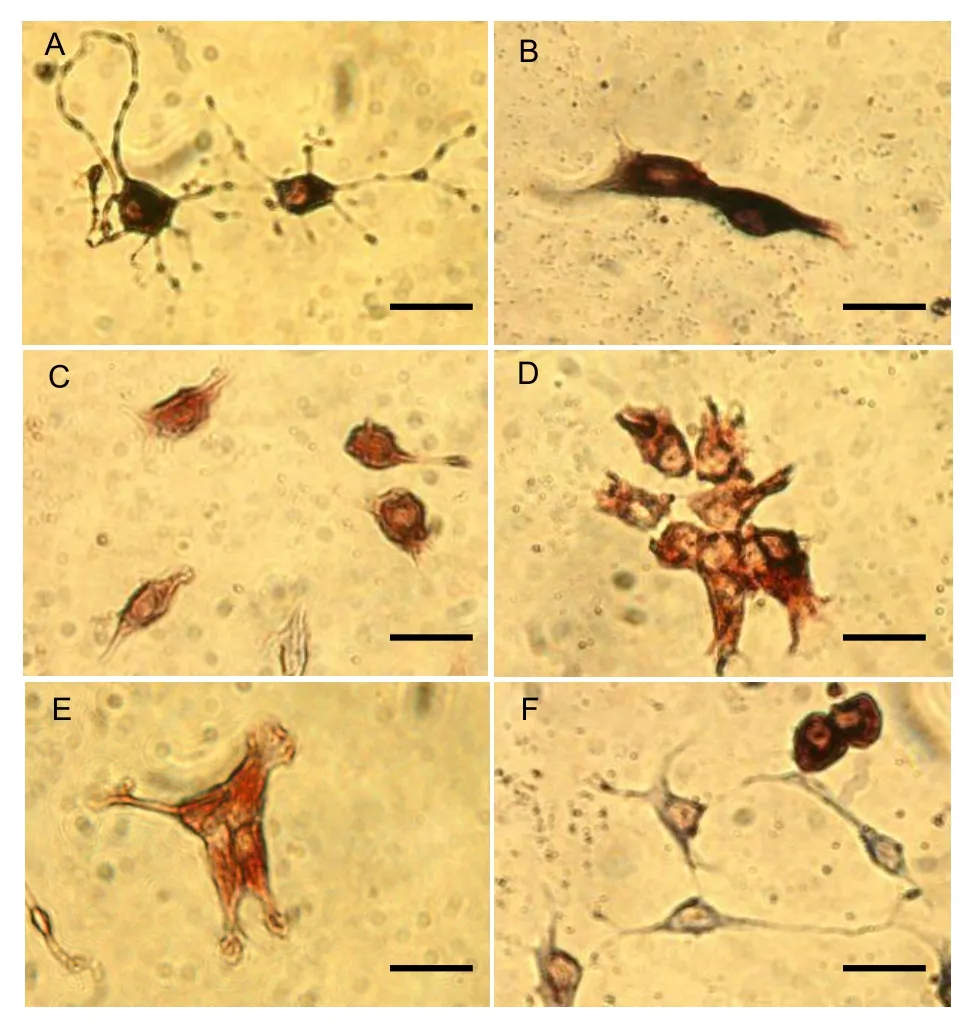
Figure 3 Neural stem cells induced by bone morphogenetic protein 4 for 7 d, as shown by choline acetyltransferase (ChAT) and nestin immunocytochemistry (scale bars: 10 μm). (A) ChAT-positive cells showing cytoplasmic staining and typical neuronal morphological characteristics, including slightly stained large, round nuclei with a clear nucleolus, and processes of various numbers and sizes originating from the cell soma. (B) ChAT-positive cells in the dividing stage. (C, D) Nestin-positive cells with a round or precursor cell-like appearance and cytoplasmic staining. (E) Nestin-positive cells in the dividing stage, with the cell soma incompletely extended. (F) Cells do not express ChAT or nestin, but exhibit neuronal morphological characteristics.
Indirect immunofluorescence staining with ChAT antibody: To further validate the effects of BMP-4 on cholinergic differentiation of rat brain-derived NSCs, the BMP-4 and control groups were cultured for 7 days and indirect immunofluorescence staining was performed with a ChAT-specific antibody. In the BMP-4 group, many cells expressed ChAT, and some cells exhibited marked neuronal morphological characteristics,i.e., large and round transparent nuclei, each cell had one or several processes, and the cells were interconnected by processes (Figure 4A). In the control group, a small number of cells expressed ChAT (Figure 4B). These findings demonstrated that BMP-4 significantly increased the proportion of ChAT-positive cells derived from NSCs.

Figure 4 Neural stem cells from the adult rat hippocampus, striatum, third ventricle, lateral ventricle bands, and subventricular bands at 7 d after culture in the presence of bone morphogenetic protein 4 (BMP-4), as shown by indirect immunofluorescence choline acetyltransferase (ChAT) staining (scale bars: 10 μm). (A) In the BMP-4 group, the majority of cells strongly express ChAT and some cells exhibit neuronal-like processes. (B) In the control group, only a few cells exhibit weak ChAT expression.
Flow cytometry detection with fluorescein isothiocyanate (FITC) labeling: to determine the percentage of rat brain-derived NSCs that differentiate into cholinergic cells as a result of BMP-4 induction, the BMP-4 and control groups were cultured for 7 days, followed by FITC labeling and flow cytometry detection. Results revealed that the proportion of ChAT-positive cells was significantly greater in the BMP-4 group compared to the control group (15.99±1.42%vs. 7.03±2.25%,P<0.05) (Figure 5).
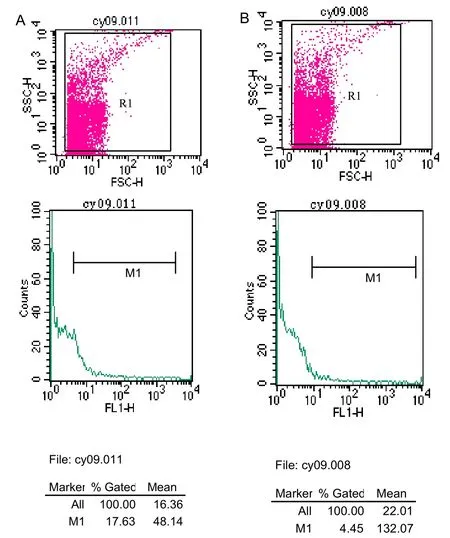
Figure 5 Detection of choline acetyltransferase expression (fluorescein isothiocyanate labeling) in neural stem cells from the adult rat hippocampus, striatum, third ventricle, lateral ventricle bands, and subventricular bands following induction of bone morphogenetic protein 4 (BMP-4), as detected by flow cytometry. (A) BMP-4 group. (B) Control group.
These findings suggested that BMP-4 increased cholinergic differentiation in rat brain-derived NSCs.
In vivo experiments Quantitative analysis of experimental animals
A total of 20 male Sprague-Dawley rats at 20 months of age randomly received a BMP-4 (BMP-4 group) or physiological saline (control group) injection into the right hippocampus. All 20 rats were included in the final analysis.
Cholinergic expression in NSCs from the adult rat hippocampal dentate gyrus
To determine oriented induction of BMP-4 in NSC differentiation in the rat hippocampal dentate gyrus, ChAT immunocytochemistry was performed at 14 days after BMP-4 injection. Results revealed ChAT-positive neurons in the hippocampal CA1, CA2, and CA3 regions, as well as the dentate gyrus. These neurons exhibited an ovoid, spindle-shaped, or polygonal appearance, with ChAT expression in the processes and cytoplasm, as well as a large soma and large, round nucleus. Hematoxylin-eosin was used to stain the nuclei (Figure 6).

Figure 6 Effects of intraventricular injection of bone morphogenetic protein 4 on cholinergic cells in the rat hippocampal dentate gyrus as shown by immunohistochemistry (scale bar: 10 µm). Choline acetyltransferase-positive cells in the hippocampal dentate gyrus exhibit an ovoid, spindle-shaped, or polygonal appearance, with expression in the processes and cytoplasm, a large soma, and large, round nucleus, as well as clear nucleolus.
The total area of ChAT-positive cells in the left and right hippocampal dentate gyrus was compared between the BMP-4 and control groups. In the BMP-4 group, the total area of ChAT-positive cells in the right hippocampal dentate gyrus was significantly greater than in the left dentate gyrus (198.73±9.85 µm2vs.161.10±8.97 µm2,P<0.01). In the control group, there was no significant difference in total area of ChAT-positive cells between the left and right hippocampal dentate gyrus (146.32±7.21 µm2vs. 140.23±7.20 µm2,P>0.05). Moreover, the total area of ChAT-positive cells in the right dentate gyrus of the BMP-4 group was significantly greater than the control group (P<0.01). There was no significant difference in ChAT-positive cell area in the left dentate gyrus between BMP-4 and control groups (P>0.05). These findings demonstrated that BMP-4 injection into the rat hippocampal dentate gyrus increased cholinergic expression in the hippocampus.
DISCUSSION
Directed differentiation of NSCs is a rapidly growing area in research related to nervous system regeneration and repair[13-17]. Cholinergic neurons in the central nervous system participate in learning and memory[18-19], and cholinergic differentiation of NSCs has been a focus in learning and memory research, as well as Alzheimer’s disease therapeutic strategies. Strong evidence exists that some BMPs, such as BMP-2, BMP-6, and BMP-7, influence nervous system development and exhibit a neurotrophic role[20-23]. The present study investigated the role of BMP-4 in inducingin vivoandin vitrocholinergic differentiation of NSCs.
In vitroexperiments demonstrated that cells harvested from the adult hippocampus, striatum, third ventricle, lateral ventricle bands and subventricular bands expressed nestin. According to results from double immunocytochemistry with nestin and ChAT antibodies, indirect immunofluorescence with ChAT antibody, and flow cytometry detection with FITC labeling, the addition of BMP-4 in culture medium induced cholinergic differentiation in 16%-18% of adult rat brain-derived NSCsin vitro. However, only 7%-8% of rat brain-derived NSCs differentiated into cholinergic neurons without exposure to BMP-4. These results suggested that BMP-4 induced cholinergic differentiation of NSCs culturedin vitro.
In addition, BMP-4 was injected into the adult rat hippocampal dentate gyrus, a brain region rich with cholinergic cells and involved in learning and memory. Results also demonstrated that the ChAT-positive cell area was greater in the BMP-4 group than in the control group. At 14 days after BMP-4 injection, there were significantly more ChAT-positive cells in the hippocampal CA1, CA2, CA3 regions, as well as the dentate gyrus, on the right side compared to the left side. However, there was no significant difference in ChAT-positive cell total area in the hippocampal dentate gyrus between the left and right sides in the control group. Moreover, the ChAT-positive cell total area in the right hippocampal dentate gyrus was greater in the BMP-4 group than in the control group. These findings demonstrated that BMP-4 injection into the adult rat hippocampal dentate gyrus induced cholinergic expression and increased the number of cholinergic cells in this brain region. BMP-2, BMP-4, and BMP-9 have been shown to be associated with cholinergic nervous system development[10-12]. However, results from the present study did not demonstrate that BMP-4-induced cholinergic expression correlates with increased NSC differentiation in the adult rat hippocampus. Further studies are needed to determine whether BMP-2, BMP-4, or BMP-9 predominates in inducing cholinergic differentiation.
Results from the present study demonstrated that BMP-4 inducedin vitrocholinergic differentiation of NSCs and increased the number of cholinergic cells in the adult rat hippocampal dentate gyrus. These results suggested that BMP-4 and BMPs promote cholinergic differentiation, which provides novel methods for treating neurodegenerative diseases, as well as learning and memory impairments and Alzheimer’s disease.
MATERIALS AND METHODS
Design
Anin vitro,comparative, observational experiment, and randomized, controlled, animal experiment.
Time and setting
This study was performed at the Laboratory of Cell Biology, Institute of Geriatrics, General Hospital of Chinese PLA, China.
Materials
Thirty 2-month-old, healthy, male, clean-grade, Sprague-Dawley rats, weighing 180 g, and twenty 20-month-old, healthy, male, clean-grade, Sprague-Dawley rats, weighing 500 g, were provided by the Academy of Military Medical Sciences of Chinese PLA (permission No. SCXK (military) 2002-001). The experimental protocol was in accordance with theGuidance Suggestions for the Care and Use of Laboratory Animalsformulated by the Ministry of Science and Technology of China[24].
Methods
In vitro experiments
Isolation, culture, and identification of NSCs: Following anesthesia by chloral hydrate, the entire brain of 2-month-old rats was collected. Tissues were collected from the hippocampus, striatum, third ventricle, lateral ventricle bands, and subventricular bands. The tissues were then mixed on the flat plate, chopped into 1-mm3pieces, and digested with 0.133% trypsin, 0.07% hyaluronidase, and 0.02% kynurenic acid for 1 hour at 37 °C. The reaction was terminated with culture medium containing 10% fetal bovine serum, followed by centrifugation at 1 000 r/min for 5 minutes. The precipitates were re-suspended with modified serum-free DMEM/F12 (1: 1) culture medium (containing B27, epidermal growth factor, basic fibroblast growth factor, N2, B27, and glutamine, each 20 mL), inoculated into 25-cm2culture flasks, and cultured at 37 °C in a CO2environment. After 24 hours, the cells were plated on a 35-mm poly-l-lysine pre-coated dish. Immunocytochemistry for nestin, glial fibrillary acidic protein (GFAP), and neurofilament (NF)160 (Chemicon, Santa Cruz, CA, USA) was performed when the cells were adherent.
Induced NSC differentiation: After 24 hours in culture, a portion of the above-mentioned cells were cultured with 10 ng/mL BMP-4 (Sigma, St. Louis, MO, USA) (BMP-4 group), and the remainder were cultured with original culture medium (control group). Cell morphology and movement were observed daily through the use of an inverted optical microscope (Olympus, Tokyo, Japan), and images were collected.
Double immunocytochemistry: At 7 days after induced culture, the cells were fixed with 4% paraformaldehyde for 1 hour, followed by blocking with normal goat serum for 20 minutes, incubation with mouse anti-ChAT polyclonal antibody (1: 200; Wuhan Boster, Hubei Province, China) or mouse anti-nestin polyclonal antibody (1: 6 000; Wuhan Boster) at 4 °C overnight, incubation with biotinylated goat anti-mouse IgG (1: 100; Wuhan Boster) at room temperature for 20 minutes, development with 5-bromo-4-chloro-3-indoyl phosphate/nitroblue tetrazolium and 3-amino-9-ethylcarbazol, and mounting with water-soluble mounting medium. Through the use of an optical microscope, ChAT-or nestin-positive cells were quantified.
Indirect immunofluorescence staining with ChAT antibody: At 8 days after induced culture, cells from BMP-4 and control groups were plated on a 35-mm poly-l-lysine pre-coated dish and indirect immunofluorescence staining was performed when the cells were adherent. The cells were incubated with mouse anti-ChAT polyclonal antibody (1: 200) at 4 °C overnight, followed by biotinylated goat anti-mouse IgG (1: 100; Wuhan Boster) at room temperature for 25 minutes. Phosphate buffered saline was used in place of the primary antibody and served as the negative control. Following addition of the streptavidin-biotin complex FITC (1: 100; Wuhan Boster), the cells were incubated at room temperature for 25 minutes. Finally, ChAT-positive cells were quantified through the use of a fluorescence microscope (Olympus).
Detection of NSC differentiation using flow cytometry and FITC labeling: Three portions of suspended cells from the BMP-4 and control groups, respectively, were adjusted to 1×107/mL. A 50-µL cell suspension from each group was incubated with rabbit anti-ChAT polyclonal antibody (1: 200; Wuhan Boster) at 4 °C for 30 minutes, washed twice with phosphate buffered saline, centrifuged, incubated with goat anti-rabbit IgG (1: 100; Wuhan Boster) or SABC-FITC (1: 100; Wuhan Boster) at 4 °C for 30 minutes, and mounted. Primary antibody was not used for the negative control. FITC-labeled cells were detected by flow cytometry (Becton-Dickinson, San Jose, CA, USA).
In vivo experiments
Following anesthesia, through the use of a brain stereotaxic instrument (Narushige Group, Tokyo, Japan), 1 µL BMP-4 (10 µg/mL) was injected into the right hippocampal dentate gyrus (anterior-posterior -3.2 mm, right lateral 2.2 mm, and ventral 3.2 mm[25]) in the BMP-4 group; the same volume of physiological saline was injected into the identical region in the control group. Immunohistochemistry: At 14 days after intraventricular injection of BMP-4, anesthesia and subsequent perfusion of 4% paraformaldehyde was performedviathe right auricle, and the rat brains were harvested. A brain tissue block was taken, spanning 5-mm anterior to 5-mm posterior from bregma, fixed in 10% paraformaldehyde, paraffin-embedded, and successively sliced into 5-µm thick sections. ChAT immunohistochemistry was then performed, and the sections were mounted with neutral resin. Under an optical microscope, five adjacent visual fields from the left and right hippocampal dentate gyrus from each sample were taken for image capture.
Through the use of an Image-prog-plus image analysis system (Media Cybernetics, Bethesda, MD, USA), the total ChAT-positive cell area in each visual field was determined, and the mean area was calculated.
Statistical analysis
All data were statistically processed using STATA software (STATA Corporation, Texas, USA) and were expressed as Mean±SD. Cell positive rate was compared between two groups using the chi-square test. Measurement data were compared using thet-test. A level ofP<0.05 was considered statistically significant.
Author contributions:Yan Chang was responsible for study concept, design, data acquisition, integration, and analysis, statistical analysis, and writing of the manuscript. Yilong Xue was in charge of study concept, design, technical guidance, writing and authorization of the manuscript. Shuilong Guo contributed to data supporting and statistical analysis. Jingkun Pan, Lei Tian, Xin Cui, and Yun Luo provided technical supports.
Conflicts of interest:None declared.
Ethical approval:Full ethical approval was obtained from the Animal Ethics Committee of General Hospital of Chinese PLA.
[1] Maciaczyk J, Singec I, Maciaczyk D, et al. Restricted spontaneous in vitro differentiation and region-specific migration of long-term expanded fetal human neural precursor cells after transplantation into the adult rat brain. Stem Cells Dev. 2009;18(7): 1043-1058.
[2] Salazar P, Velasco-Velázquez MA, Velasco I. GABA effects during neuronal differentiation of stem cells. Neurochem Res. 2008;33(8): 1546-1557.
[3] Goffredo D, Conti L, Di Febo F, et al. Setting the conditions for efficient, robust and reproducible generation of functionally active neurons from adult subventricular zone-derived neural stem cells. Cell Death Differ. 2008;15(12):1847-1856.
[4] Kallur T, Gisler R, Lindvall O, et al. Pax6 promotes neurogenesis in human neural stem cells. Mol Cell Neurosci. 2008;38(4): 616-628.
[5] Lopez-Coviella I, Follettie MT, Mellott TJ, et al. Bone morphogenetic protein 9 induces the transcriptome of basal forebrain cholinergic neurons. Proc Natl Acad Sci U S A. 2005; 102(19):6984-6989.
[6] Lopez-Coviella I, Mellott TM, Kovacheva VP, et al. Developmental pattern of expression of BMP receptors and Smads and activation of Smad1 and Smad5 by BMP9 in mouse basal forebrain. Brain Res. 2006;1088(1):49-56.
[7] Nonner D, Barrett EF, Kaplan P, et al. Bone morphogenetic proteins (BMP6 and BMP7) enhance the protective effect of neurotrophins on cultured septal cholinergic neurons during hypoglycemia. J Neurochem. 2001;77(2):691-699.
[8] López-Coviella I, Berse B, Krauss R, et al. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289(5477):313-316.
[9] Schnitzler AC, Mellott TJ, Lopez-Coviella I, et al. BMP9 (bone morphogenetic protein 9) induces NGF as an autocrine/paracrine cholinergic trophic factor in developing basal forebrain neurons. J Neurosci. 2010;30(24):8221-8228.
[10] Chang Y, Xue YL, Luo Y, et al. The cholinergic-inducing effect of BMP4 on rat's cerebral neural stem cells. Biaoji Mianyi Fenxi yu Linchuang. 2004;11(1):18-21.
[11] Berse B, Szczecinska W, Lopez-Coviella I, et al. Expression of high affinity choline transporter during mouse development in vivo and its upregulation by NGF and BMP-4 in vitro. Brain Res Dev Brain Res. 2005;157(2):132-140.
[12] Anitha M, Shahnavaz N, Qayed E, et al. BMP2 promotes differentiation of nitrergic and catecholaminergic enteric neurons through a Smad1-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G375-383.
[13] Vicario-Abejón C, Collin C, Tsoulfas P, et al. Hippocampal stem cells differentiate into excitatory and inhibitory neurons. Eur J Neurosci. 2000;12(2):677-688.
[14] Ling ZD, Potter ED, Lipton JW, et al. Differentiation of mesencephalic progenitor cells into dopaminergic neurons by cytokines. Exp Neurol. 1998;149(2):411-423.
[15] White PM, Morrison SJ, Orimoto K, et al. Neural crest stem cells undergo cell-intrinsic developmental changes in sensitivity to instructive differentiation signals. Neuron. 2001;29(1):57-71.
[16] Fann MJ, Patterson PH. Activins as candidate cholinergic differentiation factors in vivo. Int J Dev Neurosci. 1995;13(3-4): 317-330.
[17] Schorderet M. Alzheimer's disease: fundamental and therapeutic aspects. Experientia. 1995;51(2):99-105.
[18] Murchison D, McDermott AN, Lasarge CL, et al. Enhanced calcium buffering in F344 rat cholinergic basal forebrain neurons is associated with age-related cognitive impairment. J Neurophysiol. 2009;102(4):2194-2207.
[19] López-Coviella I, Blusztajn JK. Bone morphogenetic proteins and cholinergic neurons in the central nervous system. Rev Neurol. 2001;33(11):1054-1060.
[20] Mehler MF, Mabie PC, Zhu G, et al. Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev Neurosci. 2000;22(1-2):74-85.
[21] Engel U, Wolswijk G. Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells derived from adult rat spinal cord: in vitro characteristics and response to PDGF, bFGF and NT-3. Glia. 1996;16(1):16-26.
[22] Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313-1317.
[23] Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4): 585-595.
[24] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[25] Bao XM, Shu SY. The Rat Brain in Stereotaxic Coordinates. Beijing: People’s Medical Publishing House. 1991.
Cite this article as: Neural Regen Res. 2010;5(21):1611-1616.
Yan Chang★, Master, Attending physician, Laboratory of Cell Biology, Institute of Geriatrics, General Hospital of Chinese PLA, Beijing 100853, China
Yilong Xue, Master, Researcher, Laboratory of Cell Biology, Institute of Geriatrics, PLA General Hospital xueyl@plagh.com.cn
2010-07-08
2010-10-16 (N20100408002/WLM)
Chang Y, Pan JK, Tian L, Guo SL, Luo Y, Cui X, Xue YL. Bone morphogenetic protein 4 induces cholinergic differentiation of brainderived neural stem cells in vivo and in vitro. Neural Regen Res. 2010;5(21): 1611-1616.
www.crter.cn
www.nrronline.org
10.3969/j.issn.1673-5374. 2010.21.002
We would like to thank Professor Shaojun Liu and Doctor Qiuxia Lin from Academy of Military Medical Sciences of Chinese PLA, and Yazhuo Hu from Institute of Geriatrics, General Hospital of Chinese PLA for their technical supports.
(Edited by Zhou YF, Bu XY, Zhao GM/Song LP)
