Oxidative Desulfurization of Non-hydrotreated Kerosene Using Hydrogen Peroxide and Acetic Acid*
2009-05-15AsgharMolaeiDehkordiMohammadAminSobatiandMohammadAliNazem
Asghar Molaei Dehkordi**, Mohammad Amin Sobati and Mohammad Ali Nazem
Oxidative Desulfurization of Non-hydrotreated Kerosene Using Hydrogen Peroxide and Acetic Acid*
Asghar Molaei Dehkordi**, Mohammad Amin Sobati and Mohammad Ali Nazem
Department of Chemical and Petroleum Engineering, Sharif University of Technology, Tehran, Iran
oxidative desulfurization, kerosene, hydrogen peroxide, acetic acid, oxidation, extraction
1 INTRODUCTION
New environmental concerns necessitate low sulfur requirements in hydrocarbon based distillate fuels so that desulfurization of petroleum-derived fuels becomes an important part of refining processes. The sulfur-containing compounds not only cause an adverse effect on the quality of petroleum products by decreasing the American Petroleum Institute(API) gravity, but also are converted to SOduring combustion, being a major source of acid rain and air pollution [1-5]. Therefore, nowadays, very stringent legislation for ultra-low-sulfur fuels are being imposed on oil refineries throughout the world to reduce the sulfur content of their products to a very low limit around 0.001%-0.002% [4, 6].
At present, the conventional industrial process for removing sulfur-containing compounds from middle distillate fuels is hydrodesulfurization (HDS). To meet new sulfur standards with HDS process, operation at higher temperature and higher pressure with more active catalysts is indispensable, leading to higher investment and operating cost. Therefore, several new processes have been developed to remove these refractory sulfur-containing compounds satisfactorily. Such alternative processes include selective adsorption, biodesulfurization, oxidative desulfurization (ODS),[4, 7-9].
Among these alternative processes, the ODS process has received much attention as an alternative process for the desulfurization because of its two main advantages relative to the HDS process. The greatest advantage of ODS is its capability to carry out in the liquid phase under very mild temperature and pressure conditions. Another important feature of ODS is that the most refractory sulfur-containing compounds, which have less reactivity toward the HDS process [.. dibenzothiophene (DBT) and its alkylated derivatives], show high reactivity toward the ODS process. Besides, the ODS process is a non-hydrogen consuming desulfurization method and can be applied whenever enough hydrogen sources are not available. In the ODS process, the refractory sulfur-containing compounds are oxidized to their corresponding sulfoxides and sulfones subsequently. Then, these highly polarized products can be separated by means of solvent extraction, adsorption,[4, 6, 7, 10, 11]. Various types of oxidants and catalysts have been examined for the ODS process. Oxidants such as hydrogen peroxide [11-14], nitric acid [15, 16], nitrogen oxides [15], organic hydroperoxides,..-butyl hydroperoxide [17-21], ozone [22], air [23], potassium ferrate (K2FeO4) [24], Fenton’s reagent [25],. can be used. In the ODS process, hydrogen peroxide is the most common oxidant and is used in the presence of a catalyst such as acetic acid [3, 13, 26, 27], formic acid [11, 12], polyoxomethalate [11], Ti-HMS/TS-1 [28] and many other different solid basic catalysts. For example, alumina-supported polymolybdates [29], V2O5/Al2O3and V2O5/TiO2[30, 31], Co-Mo/Al2O3[32] as well as Mo/Al2O3[33] are typical solid catalysts used. Among different oxidation systems, hydrogen peroxide—carboxylic acids system (.. formic and acetic acid), which producesperacids, has several advantages over other oxidation systems, making it as one choice of the oxidation system. Thus, a desulfurization process based on this oxidation system has been developed and reported by Unipure Company with 1000 t per day capacity [34].
Heimlich and Wallace [13] have conducted a kinetic study on the oxidation of white oil solution of DBT using aqueous H2O2-acetic acid solution within the temperature range of 50 to 100ºC. They have studied the kinetics of DBT oxidation and then postulated a mechanism, which fits satisfactorily their own kinetic data [13]. Zannikos. [27] studied oxidative desulfurization of two kinds of high sulfur gas oil with sulfur mass contents of 0.87% and 2.4% using hydrogen peroxide and acetic acid in an inefficient manner. However, they used very high amount of acetic acid in their study (.., equal to the volume of gas oil) that negatively affects their process profit. Morever, they did not investigate the effects of operating parameters such as reaction temperature, oxidant/sulfur molar ratio (O/S), and acid/S molar ratio (acid/S) on the sulfur removal of their ODS system. Later, Shiraishi. [3] conducted a comprehensive study on the oxidative desulfurization of three kinds of gas oil with sulfur mass contents of 0.132%, 0.179% and 1.38% using hydrogen peroxide and acetic acid system. The main objective of their study was to investigate the potential of H2O2-acetic acid oxidation system for the desulfurization and denitrogenation of three different kinds of gas oil. However, they used high amounts of oxidant (..,O/Sranging from 100 to 3000) and acetic acid (..,acid/Sranging from 50 to 1500) in their study and did not clearly report the effects of operating parameters such as the reaction temperature,O/S, andacid/Son the sulfur removal of the ODS process.
Studies on the oxidative desulfurization of fuel oil in the boiling range of kerosene relative to gas oil are scarce particularly for non-hydrotreated kerosene. Therefore, in the present investigation, the oxidative desulfurization of non hydrotreated kerosene produced by an Iranian Refinery Company (Esfahan Refinery, Esfahan, Iran) with a sulfur mass content of 0.16% using hydrogen peroxide-acetic acid system was carried out in order to study the effects of various operating parameters such as reaction temperature,O/S, andacid/Son the sulfur removal of the ODS process in details. Besides, the performances of three types of alcohol (.. methanol, ethanol, and propanol) were examined in the extraction of oxidized sulfur compounds.
2 EXPERIMENTAL
2.1 Materials
All chemicals used in this investigation including acetic acid (99%), methanol, ethanol, propanol, and hydrogen peroxide with purity of 30% (by mass) were of analytical grade supplied by Merck Co. (Germany) and were used without further purification. Kerosene with the total sulfur mass content of 0.16% was obtained from an Iranian Refinery Company (Esfahan Refinery, Esfahan, Iran). The specifications of kerosene used in this investigation are summarized in Table 1.
2.2 Analytical method
The total sulfur content of original and treated kerosenes was determined using a non-dispersive X-ray fluorescence sulfur in oil analyzer (SLFA-20, Horiba), whose lower limitation of sulfur detection and repeatability are 0.002% (by mass) and 0.0015% (by mass), respectively. The test method is based on the American Society for Testing and Materials (ASTM) D-4294 standards. All total sulfur analysis was carried out in the central laboratory of Esfahan Refinery (Esfahan, Iran).
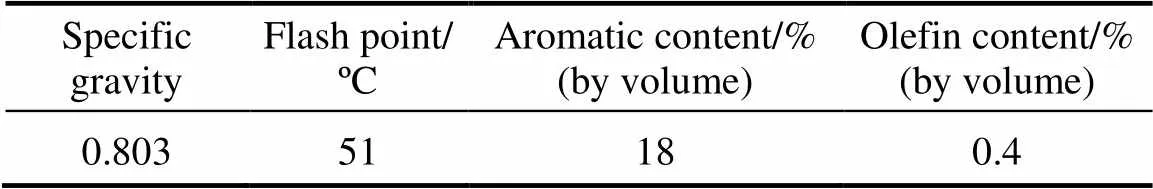
Table 1 Specifications of kerosene produced by Esfahan Refinery
Hewlett Packard 5973 gas-chromatography/mass spectrometry (GC/MS) with Hewlett Packard 6890 GC equipped with a mass selective detector and capillary column HP-5 (cross-linked 5% PhMe silicone, 60 m´0.25 mm´25 μm) was used to detect sulfur-containing compounds in kerosene qualitatively. The injector temperature was set at 280ºC with split ratio equal to 20. The initial temperature was set at 50ºC for 5 min and the temperature was then increased to 275ºC with the temperature slope of 2ºC·min-1. It is worth stressing that the present work is not aiming to study the oxidation reaction of individual sulfur-containing compounds of kerosene, but to use the oxidation reaction to reduce the total sulfur content of kerosene.
2.3 Experimental procedures
2.3.1
In each experimental run, about 750 ml of untreated kerosene with a total sulfur mass content of 0.16% was introduced into a three-necked glass reactor equipped with a condenser and a thermometer. The reactor was placed in a constant-temperature water bath with stirring. An appropriate amount of acetic acid was then added to the reactor, and the mixture was stirred continuously and heated to the desired reaction temperature. After that, an appropriate amount of hydrogen peroxide was added to the reaction mixture and this point was taken as the “zero time” of the reaction for the experimental records. The reaction time was set to be 2 h and during the course of the reaction, about 10 ml sample of the reaction mixture was withdrawn from the reactor at preset time intervals with a syringe. The progress of the oxidation reaction within the sampling bottles was immediately ceased by adding an appropriate amount of MnO2to the sampling bottles.
2.3.2
The hydrocarbon phase obtained from the reaction stage was extracted two times by means of a solution of propanol-water (volume ratio: 50%) with solvent to fuel oil volume ratio equal to 2. The general procedure for the solvent extraction was as follows: appropriate amounts of the aqueous solvent and kerosene were introduced into a separatory funnel and were shaken mechanically for 5 min at room temperature. Then, the dispersion formed was allowed to separate into two distinct phases (.., aqueous and hydrocarbon phases). The aqueous phase was removed and the hydrocarbon phase (.., treated kerosene) was washed with distilled water and dried over magnesium sulfate. After that, the total sulfur content of the treated kerosene was determined using the X-ray fluorescence technique as described in the analytical method.
For each data point, the experimental run was repeated at least two times and thus each data point was determined based on the mean value of at least two measurements with a standard deviation of 1%-3%.
3 RESULTS AND DISCUSSION
The properties of kerosene produced by Esfahan Refinery are summarized in Table 1. As can be noticed, the range of boiling point of the kerosene used is 150-272ºC. According to the Ref. [4], the main sulfur compounds of fuel oil in the boiling range of kerosene are benzothiophene and its alkylated derivatives. The GC-MS analysis confirmed the presence of benzothiophene and its alkylated derivatives such as 5-methyl bezothiophene and 3-methyl benzothiophene in kerosene. Besides, some other sulfur compounds such as 1-dodecanethiol were also observed in kerosene.
To examine the influences of various operating parameters on the performance of the ODS process, the oxidation reaction was carried out at different temperatures,O/Sandacid/S. Besides, in separate experiments, methanol, ethanol, and propanol were used in the extraction stage to find out which alcohol is more appropriate to extract oxidized sulfur-containing compounds from the treated kerosene. Moreover, the desulfurization of kerosene with simple extraction was compared with the oxidative desulfurization of kerosene in order to show the importance of oxidation stage prior to extraction stage in the final desulfurization.
3.1 Effect of reaction temperature
As can be observed from Fig. 1, a rise in the reaction temperature from 25 to 70ºC leads to a remarkable increase in the sulfur removal. Sulfur removal substantially increases from 43% at 25ºC to 74% at 60ºC, and then further to around 80% at 70ºC. This behavior is expected and can be explained by an increase in the oxidation reaction rate of sulfur-containing compounds present in kerosene due to the strong dependence of reaction rate on the reaction temperature.

Figure 1 Effect of reaction temperature on the sulfur removal
temperature/°C: ■ 25; ▲ 40; ◆ 60; ● 70
3.2 Effect of nO/nS
The influence of the oxidant (.., H2O2) to sulfur molar ratio on the oxidation of sulfur-containing compounds present in kerosene was investigated at 25ºC and 60ºC. Figs. 2 and 3 show the sulfur removal. time as a function ofO/Sat 25ºC and 60ºC, respectively.
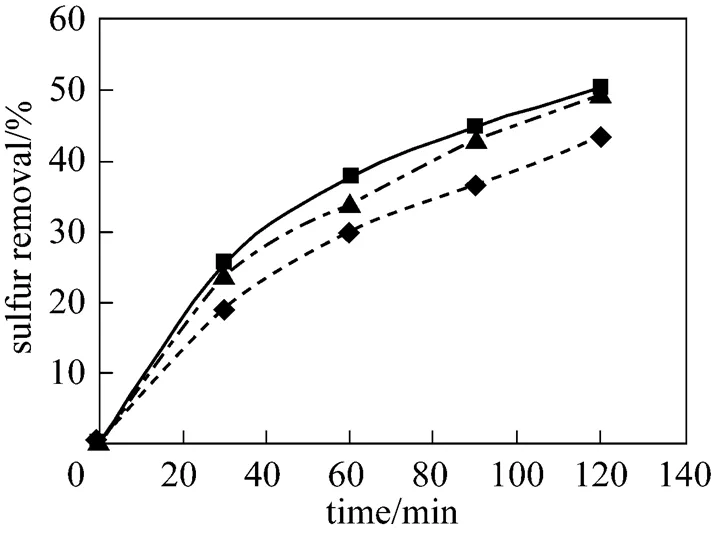
Figure 2 Effect ofO/Son the sulfur removal
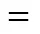
O/S:■ 8;▲ 16;◆ 23
Note that the stoichiometricO/Sis 2, however, the oxidant is usually used in excess of the stoichiometric ratio because of (1) transport limitations in two-liquid-phases reaction systems, (2) the unproductive (thermal) decomposition of H2O2to water and oxygen, and (3) possible parallel oxidation reactions such as oxidation of nitrogen-containing compounds, which are present in kerosene. Thermal decomposition of H2O2is strongly dependent on temperature, therefore, a number of oxidation runs were carried out with the varyingO/Sat two different temperatures (25ºC and 60ºC). As indicated in Fig. 2, the best results were obtained at theO/Sof 8 at 25ºC, and an increase in theO/Shigher than 8 decreases the sulfur removal slightly. In contrast with the results obtained at 25ºC, theO/Sequal 23 shows the best experimental results at 60ºC and further increase in theO/Slarger than 23 decreases the sulfur removal slightly as shown in Fig. 3. It should be mentioned that the difference between optimumO/Sat different reaction temperatures could be due to the different rates of H2O2decomposition at 25ºC and 60ºC so that at 60ºC the rate of thermal decomposition of H2O2is higher than that of 25ºC, and hence the bestO/Sfor this temperature is larger than that of 25ºC.
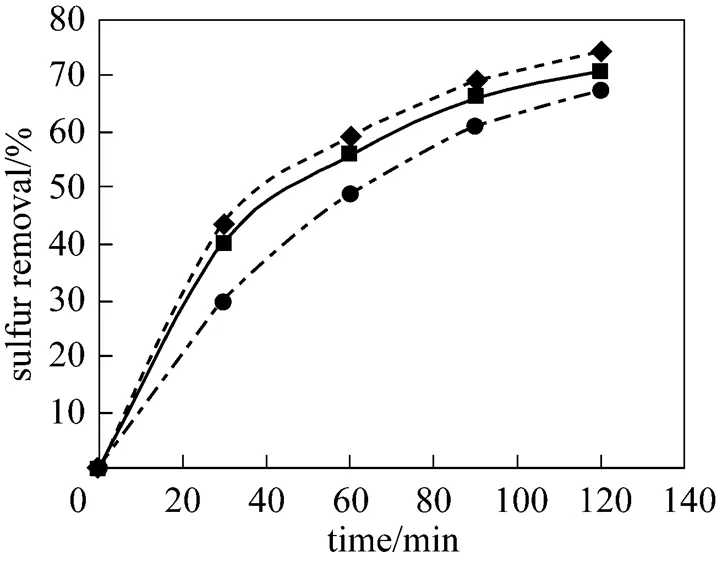
Figure 3 Effect ofO/Son the sulfur removal
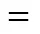
O/S:■ 8;▲ 23;◆ 31
3.3 Effect of nacid/nS
As it is well known, acetic acid catalyzes the oxidation of sulfur-containing compoundsforming peracetic acid, which acts as an oxidizing agent giving its oxygen atom to the sulfur-containing compounds present in kerosene. Peracetic acid is formedby acetic acid and hydrogen peroxide. Therefore, the oxidation reaction can be promoted by increasing acetic acid concentration. Besides, it has been found that acetic acid is itself a good extraction solvent for simultaneous extraction of the oxidized sulfur-containing compounds in reaction medium [27]. In the present investigation, it was observed that the sulfur removal increases by increasingacid/S. Figs. 4 and 5 demonstrate the variations of the sulfur removal with time as a function ofacid/Sat 25ºC and 60ºC, respectively.
This behavior is expected and can be explained by an increase in peracetic acid concentration in the reaction medium and the enhancement ofextraction of oxidized sulfur compounds in the aqueous phase of reaction medium. As an advantage for the ODS system, the aqueous solution of hydrogen peroxide and acetic acid is mostly trapped in the lower layer of two-liquid-phases system and so, can be effectively reused for the desulfurization of new feeds.
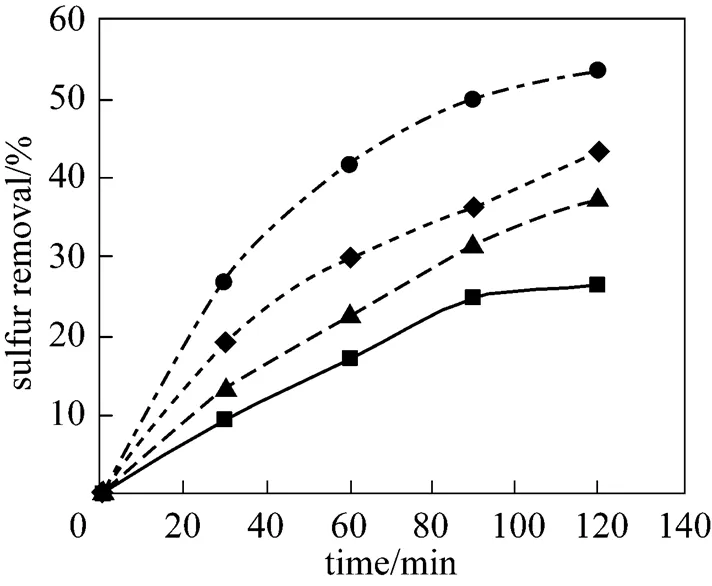
Figure 4 Effect ofacid/Son the sulfur removal
acid/S:■ 20;▲ 40;◆ 60;● 80
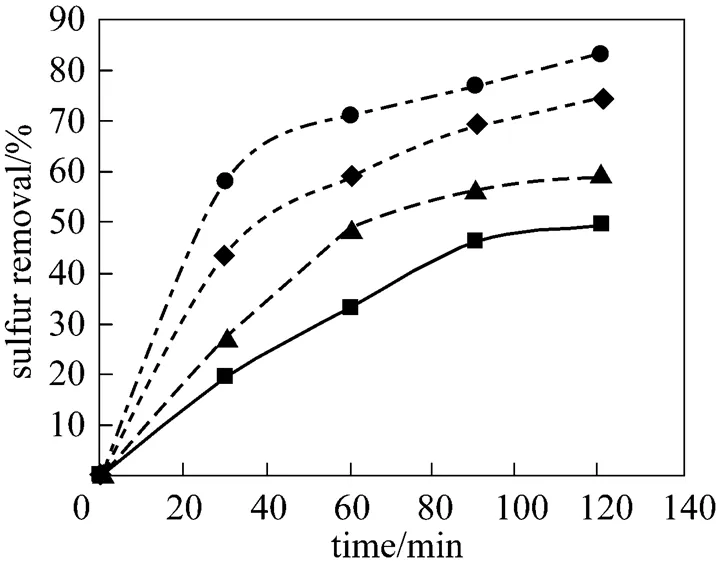
Figure 5 Effect ofacid/Son the sulfur removal
acid/S:■ 20;▲ 40;◆ 60;● 80
3.4 Effect of type of extraction solvent
After the oxidation, the oxidized sulfur-containing compounds should be removed by contacting the oxidized fuel oil with a selective extraction solvent. The solvent should be polar to be selective for the extraction of polar oxidized sulfur-containing compounds present in the oxidized kerosene. However, mere polarity considerations are not sufficient to select an appropriate extraction solvent. Other properties of the solvent to be considered are the boiling point, freezing point, surface tension, and toxicity of solvent along with the most important parameter that is the recovery percentage of fuel oil after the extraction [2]. Note that it may be possible that much of usable hydrocarbons are removed with oxidized sulfur-containing compounds by using an inappropriate solvent for the extraction stage. In the present work, aliphatic alcohols such as propanol, ethanol, and methanol were considered as the potential solvents and the selectivity of the extraction process was controlled by adding water to the solvent. Table 2 summarizes the performance capability of aforementioned alcohols for the extraction of oxidized sulfur-containing compounds after 2 h oxidation. The fuel oil recovery was about 90% in each case.
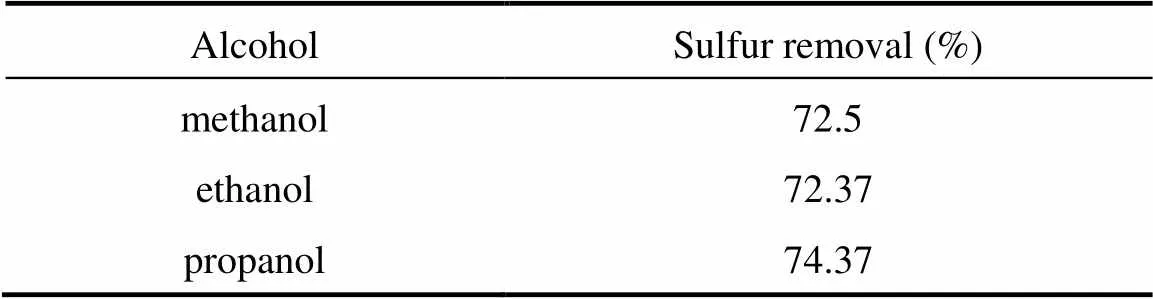
Table 2 Comparison of different alcohols [50% (by volume) alcohol-water solution] for the ODS of untreated kerosene
As can be observed, the performances of different homolog alcohols are similar, which is related to similar molecular structure and polarity of used alcohols but the performance of propanol [in 50% (by volume) propanol-water solution] was slightly better than that of the other alcohols. Therefore, propanol was selected as the target solvent for the extraction of oxidized sulfur-containing compounds and was used throughout the experimental work.
3.5 Effect of oxidation on desulfurization
As mentioned earlier, the oxidation of sulfur containing compounds changes the polarity of oxidized sulfur containing compounds dramatically and so they can be effectively separated with conventional method such as extraction. To examine the role of oxidation in the ODS process, desulfurization of non-hydro treated kerosene containing 0.16% sulfur by simple extraction process was compared with oxidation followed by extraction (.., ODS process). It was found that the simple extraction process leads to only 9% sulfur removal but the oxidation before the extraction process enhances the sulfur removal to 83.3%. These results clearly show the importance of oxidation stage in the overall ODS process.
The distillation curves of kerosene before and after ODS process were obtained according to ASTM-D86 in order to investigate whether the properties of desulfurized kerosene are negatively affected by the oxidation followed by extraction. Fig. 6 shows the distillation curves of treated kerosene with ODS and non-hydrotreated kerosene.

Figure 6 ASTM D-86 distillation curves of treated and untreated kerosene
■ untreated kerosene;▲ treated kerosene
It can be seen that the distillation characteristics were not significantly affected in the ODS process. This clearly shows that the ODS process has no significant effects on the kerosene distillation characteristics and the general properties of kerosene was preserved during the ODS treatment.
4 CONCLUSIONS
The oxidative desulfurization of untreated kerosene using H2O2-acetic acid system was studied. The influences of various operating parameters such as reaction temperature,O/S, andacid/Son the performance of the ODS process were examined. It was found that:
(1) Sulfur removal increases by increasing temperature due to an increase in oxidation reaction rate of sulfur containing compounds presented in kerosene.
(2) OptimumO/Sfor the oxidation of sulfur-containing compounds of untreated kerosene is to some extent dependent on the reaction temperature. TheO/Sof 8 and 23 gave the higher sulfur removal at 25ºC and 60ºC, respectively.
(3) Sulfur removal increases by increasingacid/Sdue to an increase in the peracetic acid concentrationand enhancement ofextraction of oxidizedsulfur containing compounds in reaction medium.
(4) Propanol showed slightly better extraction ability for the oxidized sulfur-containing compounds presented in the treated kerosene relative to other aliphatic alcohols such as methanol and ethanol.
(5) The role of oxidation in the ODS process is very important. It is obvious that the oxidation will change the properties of sulfur containing compounds in such a way that they can be readily separated. The extraction with simple solvent (.. alcohols) without oxidation is not an efficient method for sulfur removal in the desulfurization of non-hydrotreated kerosene.
(6) The maximum observed sulfur removal in the present study was 83.3%. Thus, it is important to focus on the improvement of sulfur removal by using other catalysts or oxidation systems.
(7) ASTM D-86 distillation of untreated and treated kerosene were similar. Therefore, the present ODS process has no profound effect on the distillation properties of kerosene.
ACKNOWLEDGEMENTS
.
NOMENCLATURE
S0initial sulfur concentration
acid/Sacid to sulfur molar ratio
O/Soxidant to sulfur molar ratio
S/F solvent per fuel oil volume ratio
reaction temperature, ºC
time, min
1 Horii, Y., Onuki, H., Doi, S., Mori, T., Takatori, T., Sato, H., Ookuro, T., Sugawara, T., “Desulfurization and denitration of light oil by extraction”, U.S. Pat., 5494572 (1996).
2 Gore, W., “Method of desulfurization of hydrocarbons”, U.S. Pat., 6160193 (2000).
3 Shiraishi, Y., Tachibana, K., Hirai, T., Komasawa, I., “Desulfurization and denitrogenation process for light oils based on chemical oxidation followed by liquid-liquid extraction”,...., 41, 4362-4375 (2002).
4 Song, C., Ma, X., “New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization”,..,, 41, 207-238 (2003).
5 Yen, T.F., Mei, H., Lu, S.H., “Oxidative desulfurization of fossil fuels with ultrasound”, U.S. Pat., 6402939 (2002).
6 Nie, Y., Li, C.X., Wang, Z.H., “Extractive desulfurization of fuel oil using alkylimidazole and its mixture with dialkylphosphate ionic liquids”,...., 46 (15), 5108-5112 (2007).
7 Campos-Martin, J.M., Capel-Sanchez, M.C., Fierro, J.L.G., “Highly efficient deep desulfurization of fuels by chemical oxidation”,., 6, 557-562 (2004).
8 Ali, M.F., Al-Malki, A., El-Ali, B., Martinie, G., Siddiqui, M.N., “Deep desulphurization of gasoline and diesel fuels using non-hydrogen consuming techniques”,, 85, 1354-1363 (2006).
9 Haji, S., Erkey, C., “Removal of dibenzothiophene from model diesel by adsorption on carbon aerogels for fuel cell applications”,...., 42, 6933-6937 (2003).
10 Filippis, P.D., Scarsella, M., “Oxidative desulfurization: Oxidation reactivity of sulfur compounds in different organic matrixes”,, 17, 1452-1455 (2003).
11 Te, M., Fairbridge, C., Ring, Z., “Oxidation reactivities of dibenzothiophenes in polyoxometalate/H2O2and formic acid/H2O2systems”,..,, 219, 267-280 (2001).
12 Otsuki, S., Nonaka, T., Takashima, N., Qian, W., Ishihara, A., Imai, T., Kabe, T., “Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction”,, 14, 1232-1239 (2000).
13 Heimlich, B.N., Wallace, T.J., “Kinetics and mechanism of the oxidation of dibenzothiophene in hydrocarbon solution”,, 22, 3571-3579 (1966).
14 Yu, G., Lu, S., Chen, H., Zhu, Z., “Diesel fuel desulfurization with hydrogen peroxide promoted by formic acid and catalyzed by activated carbon”,, 43, 2285-2294 (2005).
15 Tam, P.S., Kittrell, J.R., Eldridge, J.W., “Desulfurization of fuel oil by oxidation and extraction (1) Enhancement of extraction oil yield”,...., 29, 321-324 (1990).
16 Tam, P.S., Kittrell, J.R., Eldridge, J.W., “Desulfurization of fuel oil by oxidation and extraction (2) Kinetic modeling of oxidation reaction”,...., 29, 324-329 (1990).
17 Wang, D., Qian, E.W., Amano, H., Okata, K., Ishihara, A., Kabe, T., “Oxidative desulfurization of fuel oil (I) Oxidation of dibenzothiophenes using-butyl hydroperoxide”,..,, 253, 91-99 (2003).
18 Chica, A., Gatti, G., Moden, B., Marchese, L., Iglesia, E., “Selective catalytic oxidation of organosulfur compounds with-butyl hydroperoxide”,..., 12, 1960-1967 (2006).
19 Ishihara, A., Wang, D., Dumeignil, F., Amano, H., Qian, E.W., Kabe, T., “Oxidative desulfurization and denitrogenation of a light gas oil using an oxidation/adsorption continuous flow process”,..,, 279, 279-287 (2005).
20 Chica, A., Corma, A., Dómine, M.E., “Catalytic oxidative desulfurization (ODS) of diesel fuel on a continuous fixed-bed reactor”,.., 242, 299-308 (2006).
21 Prasad, V.V.D.N., Jeong, K.E., Chae, H.J., Kim, C.U., Jeong, S.Y., “Oxidative desulfurization of 4,6-dimethyl dibenzothiophene and light cycle oil over supported molybdenum oxide catalysts”,.., 9, 1966-1969 (2008).
22 Otsuki, S., Nonaka, T., Qian, W., Ishihara, A., Kabe, T., “Oxidative desulfurization of middle distillate using ozone”,...., 42, 315-320 (1999).
23 Sampanthar, J.T., Xiao, H., Dou, J., Nah, T.Y., Rong, X., Kwan, W.P., “A novel oxidative desulfurization process to remove refractory sulfur compounds from diesel fuel”,..,, 63, 85-93 (2006).
24 Liu, S., Wang, B., Cui, B., Sun, L., “Deep desulfurization of diesel oil oxidized by Fe (VI) systems”,, 87, 422-428 (2008).
25 Dai, Y., Qi, Y., Zhao, D., Zhang, H., “An oxidative desulfurization method using ultrasound/Fenton’s reagent for obtaining low and/or ultra-low sulfur diesel fuel”,.., 89, 927-932 (2008).
26 Shiraishi, Y., Hirai, T., “Desulfurization of vacuum gas oil based on chemical oxidation followed by liquid-liquid extraction”,, 18, 37-40 (2004).
27 Zannikos, F., Lois, E., Stournas, S., “Desulfurization of petroleum fractions by oxidation and solvent extraction”,.., 42, 35-45 (1995).
28 Jin, C., Li, G., Wang, X., Wang, Y., Zhao, L., Sun, D., “A titanium containing micro/mesoporous composite and its catalytic performance in oxidative desulfurization”,., 111, 236–242 (2008).
29 Garcia-Gutierrez, J.L., Fuentes, G.A., Hernandez-Teran, M.E., Murrieta, F., Navarrete, J., Jimenez-Cruz, F., “Ultra-deep oxidative desulfurization of diesel fuel with H2O2catalyzed under mild conditions by polymolybdates supported on Al2O3”,..,, 305, 15-20 (2006).
30 Cedeno-Caero, L., Navarro A., J.F., Gutierrez-Alejandre, A., “Oxidative desulfurization of synthetic diesel using supported catalysts (II) Effect of oxidant and nitrogen-compounds on extraction-oxidation process”,., 116, 562-568 (2006).
31 Cedeno-Caero, L., Gomez-Bernal, H., Fraustro-Cuevas, A., Guerra-Gomez, H.D., Cuevas-Garcia, R., “Oxidative desulfurization of synthetic diesel using supported catalysts (III) Support effect on vanadium-based catalysts”,., 133-135, 244-254 (2008).
32 Zapata, B., Pedraza, F., Valenzuela, M.A., “Catalyst screening for oxidative desulfurization using hydrogen peroxide”,., 106, 219-221 (2005).
33 Garcia-Gutierrez, J.L., Fuentes, G.A., Hernandez-Teran, M.E., Garcia, P., Murrieta-Guevara, F., Jimenez-Cruz, F., “Ultra-deep oxidative desulfurization of diesel fuel by the Mo/Al2O3-H2O2system: The effect of system parameters on catalytic activity”,..,, 334, 366-373 (2008).
34 Ito, E., Rob van Veen, J.A., “On novel processes for removing sulphur from refinery streams”,., 116, 446-460 (2006).
2009-05-05,
2009-08-27.
the R&D center of Esfahan refinery (Esfahan, Iran).
** To whom correspondence should be addressed. E-mail: amolaeid@sharif.edu
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Molecular Simulation of CO2/H2 Mixture Separation in Metal-organic Frameworks: Effect of Catenation and Electrostatic Interactions*
- Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
- Corrosion Behavior of TP316L of Superheater in Biomass Boiler with Simulated Atmosphere and Deposit
- Influence of A-type Zeolite on Methane Hydrate Formation*
- Effects of Sintering Atmosphere on the Microstructure and Surface Properties of Symmetric TiO2Membranes*
- Improvement of Isomerization Process of Crude Isoamylene with Tertiary-amyl-alcohol Addition
