Efficient and Comprehensive Utilization of Hemicellulose in the Corn Stover*
2009-05-12GAOPengfei高鹏飞FANDaidi范代娣LUOYan骆艳娥MAXiaoxuan马晓轩MAPei马沛HUIJunfeng惠俊峰andZHUChenhui朱晨辉
GAO Pengfei (高鹏飞), FAN Daidi (范代娣),**, LUO Yan’e (骆艳娥), MA Xiaoxuan (马晓轩), MA Pei (马沛), HUI Junfeng (惠俊峰) and ZHU Chenhui(朱晨辉)
Efficient and Comprehensive Utilization of Hemicellulose in the Corn Stover*
GAO Pengfei (高鹏飞)1,2,3, FAN Daidi (范代娣)1,2,3,**, LUO Yan’e (骆艳娥)1,2,3, MA Xiaoxuan (马晓轩)1,2,3, MA Pei (马沛)1,2,3, HUI Junfeng (惠俊峰)1,2,3and ZHU Chenhui(朱晨辉)1,2
1Shaanxi Key Laboratory of Degradable Biomedical Materials, Northwest University, Xi’an 710069, China2Shaanxi R&D Center of Biomaterial and Fermentation Engineering, Northwest University, Xi’an 710069, China3College of Chemical Engineering, Northwest University, Xi’an 710069, China
Pretreatment of the corn stover powder by dilute sulphuric acid (solid-liquid ratio 1︰20) at 130°C for 30 min was carried out with 89.09% of the hemicellulose removed. After filtration, the xylose-rich corn stover pretreatment liquid, whose fermentable sugar was from hemicellulose hydrolysis only, consisting of 81.16% xylose and 15.27% glucose, was used to cultivate genetic recombinantBL21 with human-like collagen (HLC) expression enhanced by 50.00% and 63.71% xylose consumption.
corn stover,BL21, human-like collagen, combined sugar
1 INTRODUCTION
Biofuels, in particular ethanol, are touted as a partial solution both to the world’s mushrooming energy demands and to the challenge of reducing greenhouse gas emission from fossil fuels [1]. Bioethanol, 40% of which comes from crops, currently accounts for more than 94% of global biofuel production [2, 3]. It has already pointed out that production of bioethanol by lignocellulose is more sensible to human beings than other forms of biomass [4-6]. On the global scale, lignocellulose can produce up to 442 billion litre bioethanol per year, while the total potential bioethanol production can reach up to 491 billion litre per year [7, 8]. Moreover, the American law has already been signed by US President Bush that from 2016 refiners must switch to cellulosic ethanol and other advanced biofuels that do not rely on corn [9].
Corn stover is a kind of lignocellulosic biomass—the most abundant and reproducible resource on Earth, which involves agriculture residues (corn stover and wheat straw), wood, grass, sawdust,[5, 6]. Recent years’ researches and industrial practices suggest that corn stover is favored in the bioethanol production process. In the Union Address by US President Bush in 2006, the bioethanol production by corn stover will be favored by the government [4]. In China’s 9th and 10th Five-Year plan, the production of bioethanol from corn stover is highlighted [4]. The productivity of bioethanol from corn stover can reach up to 375 L·t-1by the technology of National Renewable Energy Laboratory (NREL), which is a comparatively practical approach, including dilute-acid pretreatment, enzymatic hydrolysis and fermentation of ethanol [4]. The basic structure of lignocellulose consists of cellulose (C5H10O5), hemicelluloses such as xylan (C5H8O4), and lignin [C9H10O3·(OCH3)0.9-1.7][10, 11]. The first step is size reduction and pretreatment when converting the corn stover to bioethanol [12]. Pretreatments alter or remove the structural and compositional impediments of the lignocellulose such as lignin and hemicellulose [6, 13]. It has been pointed out that the xylose yield could reach up to 75%-90% by dilute-acid pretreatment (the most widely used and studied approach) [5, 14, 15]. Unfortunately, the cellulases are notoriously susceptible to feedback inhibition by pentose (xylose included) which is yielded during pretreatment and cannot be utilized by the ethanol fermentation strain.[16, 17]. Moreover, the poor utilization of the pentose due to the hydrolysis of the hemicellulose leads to waste and cost soaring [4]. Therefore, it is necessary and compulsory to remove and utilize the xylose: this article discusses a novel way of xylose exploitation.
2 EXPERIMENTAL
2.1 Dilute-acid pretreatment
Add corn stover powder (size dimension less than 3 mm) to 0.75% (by volume) dilute sulphuric acid at solid-liquid ratio of 1︰20; then autoclave (Autoclave, Hirayama HV-50, Japan) at 130°C for 30 min [5].
2.2 Preparation of pretreatment culture medium (PCM) for Escherichia coli BL21
Settled solution was obtained after filtration of the pretreated system where both the corn stover powder and dilute sulfuric acid were included. The reduced sugar concentration of the solution was adjusted to 10 mg·ml-1by distilled water (Spectrophotometer, UNICO UV-2600A, USA), while the pH of the solution was adjusted to 6.80 with 3 mol·L-1NaOH solution (pH-meter, Mettler Toledo FE20, Switzerland) simultaneously. Then 0.50 g sodium chloride, 5.00 g peptone (Oxoid, LP0021, UK) and 2.50 g yeast extract powder (Merck VL684026, USA) were added to the 500 ml solution which was filtrated again by the filter paper. The total 500 ml culture medium was divided equally to 10 shake flasks (volume 250 ml) with 50 ml culture medium each. Autoclave sterilization at 121°C for 20 min was conducted for preparation.
2.3 Optimization of the PCM for Escherichia coli BL21
The PCM compositions were optimized by response surface methodology. The factors that had significant effects on the growth of the strain were selected by Plackett-Burman design and the path of the steepest ascent was employed to approach the optimum region of response. Central composite design (CCD) and response surface methodology (RSM) were employed to investigate the interaction of the variables and to ascertain the optimal values of the factors, which finally led to the maximum production of single cell protein human-like collagen (HLC). All the work was aided by the software “Minitab, release of Minitab Inc” [18, 19].
2.4 Cultivation of the genetic recombinant Escherichia coli BL21 in PCM
Cultivation was conducted in a LB shaking flask (volume 250 ml) with a medium load of 50 ml under 170 r·min-1at 34°C for 12 h to obtain the seed culture (Incubator, Zhicheng ZHWY 2112B, China). After 12h cultivation, the PCM (10 shaking flasks of 250 ml volume each) and its control (10 shaking flasks of 250 ml volume each) were inoculated with 2 ml of the inoculum from the seed culture. Cultivation was carried out under 170 r·min-1and at 34°C for the first 10 hours, then 42°C for the next 3 hours and 39°C for the final 7 hours to make this thermo induction strain express HLC.
2.5 Determination of the proteins
The PCM and its control were freezing-offcentered (Centrifugal, Thermo KeyWrite-DTM, USA) after 20 hours of cultivation at a speed of 6000 r·min-1for 20 min. After removing the supernatant, the precipitation was washed twice until the eluent was tested without protein. Dry mass (Ultra cold refrigerator, Revco ULT1386-3, USA; Freeze dryer, Labconco Freezone 6, UK) was obtained by freeze-drying. Then, the isocratic quantum of each was taken and dissolved in 100 ml water. After ultrasonic wall breaking, freezing centrifugation was conducted at a speed of 7000 r·min-1for 20 min to obtain the intracellular protein. Through purification, HLC was acquired. Both the intracellular total protein (ICTP) and HLC were determined by the biuret method [20] (Spectrophotometer, UNICO UV-2600A, USA).
2.6 Analysis of the sugars
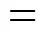
3 RESULTS AND DISCUSSION
3.1 Pretreatment
Total reduced sugar yield was 0.27 g·g-1(based on dry stuff) (Spectrophotometer, UNICO UV-2600A, USA) [21]. It was shown that xylose accounted for 81.16% of the total sugar while the glucose only 15.27% by the HPLC analysis, which denoted that the xylose yield was 0.22 g·g-1. The compositions of unpretreated corn stover and dilute-acid pretreated corn stover were assessed [22]. Dilute-acid pretreatment removed 89.09% of the hemicellulose (Fig. 1). In addition, Demirbas came up with two steps for the pretreatment: the first step was conducted under mild conditions to recover the pentose and the second step was carried out under harsher condition for the hexose, since it was more rapid and easier for the pentose to degrade than the hexose [23]. Amidon and Liu [24] offered the biorefinery concept including hot water extraction, hydrolysis, separation, fermentation, pulping, burning,., where they stated that hemicellulose was the most easily separation component and formed the bulk of hot-water extractions. Moreover, Danny. [25] stated that the ferulic acid esterase treatment before cellulase treatment significantly improved the dry mass loss and release of phenolic acids and sugars in most fractions over cellulase alone.

Figure 1 Compositions of the corn stover■ unpretreated;□ dilute-acid pretreated
3.2 Optimization of the PCM for Escherichia coli BL21
For response surface experiments (Fig. 2), according to Plackett-Burman design and the path of the steepest ascent, Table 1 was founded to optimize the two major significant factors, the concentrations of peptone and yeast extract, which had positive effects on the growth ofBL21 during the cultivation. The other compositions remained the same as mentioned in Section 2.2 [19]. The analysis suggested the following response surface,

through which the optimal concentrations of the peptoneand yeast extract were determined as 9.98 mg·ml-1and5.01 mg·ml-1respectively, demonstrating that the prescription in Section 2.2 was already at its optimal level
Figure 2 Effects of peptone and yeast extract on the HLC production

Table 1 Response surface experiment design
3.3 Cultivation of the genetic recombinant Escherichia coli BL21 in the PCM
With regard to the utilization of the xylose, genetically modifiedcapable of utilizing xylose was involved in the researches conducted by Zaldivar. [26, 27], while the strategy of natural substrate utilization was involved, which included taking advantage of strains with the inherent xylose- exploiting ability likeandto exploit xylose [4]. All mentioned above indicated thatcould exploit xylose.
Genetic recombinantBL21 was preserved by the laboratory with thermo induction, which could be cultivated at a high-density and produce single cell protein HLC.
3.3.1
The growth curve was determined by assay of OD600once an hour (Spectrophotometer, UNICO UV-2600A, USA) (Fig. 3). After 20 hours of cultivation, HLC was assayed. The control remained the same as the PCM except that the fermentable sugar was the analytical glucose at the same concentration of 10 mg·ml-1not the combined sugar (derived from the corn stover pretreatment liquid), 81.16% of which was xylose while glucose was only 15.27% of the PCM. Apparently, the strain grew faster in the control than in the PCM at first, especially for the first four hours since the strain could take in glucose easier. However, from the 10th hour to the 17th hour the strain grew faster in the PCM than in the control.

Figure 3 Growth curves of.BL21 in the PCM and its control■ control;▲ PCM
After the 13th hour the strain in the control seemed to “halt”. From the 15th hour, OD600was higher in the PCM than in its control. Such a scenario probably resulted from the high concentration of the glucose contained in the control, triggering the drastic growth of the strain and accumulation of the harmful metabolic products, which finally in return decreased the growth of the strain [28]. The exhaustion of the nutrients due to the drastic growth finally aggravated the situation [28]. In contrast to the control, the strain in the PCM kept a stable and gradual-ascending growth since in the PCM the fermentable sugar was combined with a minority of glucose and a majority of xylose. Low glucose concentration precluded the defects due to the high glucose concentration in the control, which finally led to a relative predominant environment for the strain; thus we think that this combination sugar of xylose and glucose merits further investigation.
3.3.2
Reduced sugar concentration of the PCM and its control were assayed once every hour (Spectrophotometer, UNICO UV-2600A, USA) (Fig. 4). The consumption of the sugar corresponded to the growth of the strain. In the first ten hours, the strain in the control consumed much more sugar than in the PCM, which matched the drastic growth in the control well (Fig. 3). However, from the 10th to 20th hour, the situation reversed that the sugar consumption in the control hit to state of “stasis”, which suited the “halt” scenario in Fig. 3 as well. At the 20th hour when the cultivation terminated, xylose contained in the PCM was 63.71% consumed by the strain.

Figure 4 Concentrations of sugars in the media at different time■ xylose in PCM;◆ glucose in PCM;▲ glucose in control
3.3.3
The concentrations of ICTP and HLC were 2.13 mg·ml-1and 0.12 mg·ml-1respectively for the PCM, while 2.09 mg·ml-1and 0.08 mg·ml-1respectively for the control (Fig. 5). Single cell protein HLC expression was enhanced by 50.00%, which indicated thatBL21 could utilize xylose derived from the corn stover pretreatment liquid.

Figure 5 Protein expressions in the PCM and its control 1—ICTP in control; 2—ICTP in PCM; 3—HLC in control; 4—HLC in PCM
Based on the experiments, we can come to following conclusions. An excellent pretreatment result can be obtained by a dilute acid pretreatment combined with high pressure and temperature with the reduced sugar yield of 0.27 g·g-1(based on dry stuff) and xylose yield of 0.22 g·g-1, and 89.09% of the hemicellulose was removed. Moreover, genetic recombinantBL21 can assimilate xylose with 63.71% of the xylose absorbed, and the expression of single cell protein HLC in PCM is enhanced by 50.00% in comparison to the control.
NOMENCLATURE
concentration of peptone, mg·ml-1
concentration of yeast extract, mg·ml-1
1 Editorial, “Bioethanol needs biotech now”,.., 24, 725 (2006).
2 International Risk Governance Council (IRGC), “Governing the risks and opportunities of bioenergy”, IRGC’s bioenergy Project, Geneva, Switzerland (2007).
3 Dufey, A., “Biofuels production, trade and sustainable development: emerging issues”, International Institute for environment and development, London, UK (2006).
4 Qu, Y.B., “Industrialization of cellulosic ethanol”,.., 19, 1098-1108 (2007).
5 Balata, M., Balata, H., Cahide, Ö.Z., “Progress in bioethanol processing”,..., 34, 551-573 (2008).
6 Sun, Y., Cheng, J.Y., “Hydrolysis of lignocellulosic materials for ethanol production: A review”,.., 83, 1-11 (2002).
7 Bohlmann, G.M., “Process economic considerations for production of ethanol from biomass feedstocks”,.., 2, 14-20 (2006).
8 Kim, S., Dale, B.E., “Global potential bioethanol production from wasted crops and crop residues”,, 26, 361-375 (2004).
9 Editorial, “Forward with biofuels”,, 451, 865-866 (2008).
10 Demirbas, A., “Estimating of structural composition of wood and non-wood biomass samples”,, 27, 761-767 (2005).
11 Arın, G., Demirbas, A., “Mathematical modeling the relations of pyrolytic products from lignocellulosic materials”,, 26, 1023-1032 (2004).
12 Graf, A., Koehler, T., “Oregon cellulose-ethanol study: An evaluation of the potential for ethanol production in Oregon using cellulose based feedstocks”, Oregon Dept. of Energy, Oregon, USA (2000).
13 Mosier, N., Wyman, C., Dale, B., Elander, R., Holtzapple, Y.Y.L.M., Ladisch, M., “Features of promising technologies for pretreatment of lignocellulosic biomass”,.., 96, 673–686 (2005).
14 Hamelinck, C.N., van Hooijdonk, G., Faaij, A.P.C., “Prospects for ethanol from lignocellulosic biomass: techno-economic performance as development progresses”, In: Scientific Report-NWS-E-2003-55, Utrecht, Netherlands (2003).
15 Hamelinck, C.N., van Hooijdonk, G., Faaij, A.P.C., “Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term”,, 28, 384-410 (2005).
16 Óscar, J.S., Carlos, A.C., “Trends in biotechnological production of fuel ethanol from different feedstocks”,.., 99, 5270-5295 (2008).
17 Jeffries, T.W., Jin, Y.S., “Ethanol and thermotolerance in the bioconversion of xylose by yeasts”,..., 47, 221-268 (2000).
18 Li, C., Bai, J.H., Cai, Z.L., Fan, O.Y., “Optimization of a structural medium for bacteriocin production byusing response surface methodology”,.., 93, 27-34 (2002).
19 Qin, J.Y., Xiao, Z.J., Ma, C.Q., Xie, N.Z., Liu, P.H., Xu, P., “Production of 2,3-butanediol byusing glucose and ammonium phosphate”,...., 14, 132-136 (2006).
20 Li, J.W., Xiao, N.G., Yu, R.Y., Yuan, M.X., Chen, L.R., Chen, Y.H., Chen, L.T., Principle and Method in the Biochemistry Experiment, Peking University Press, Beijing, 165 (2003). (in Chinese)
21 Chen, Y.Q., Biochemical Experiment Method and Technology, Science Press, Beijing (2002). (in Chinese)
22 Ma, X.J., Li, H.L., Liu, L.P., Production and Application Technology of Fuel-Ethanol, Chemical Industry Press, Beijing (2007). (in Chinese)
23 Demirbas, A., “Progress and recent trends in biofuels”,..., 33, 1-18 (2007).
24 Amidon, T.E., Liu, S.J., “Biorefinery: Conversion of woody biomass to chemicals and energy”, In:International Conference on Biomass Energy Technologies, Guangzhou (2008).
25 Danny, E.A., Morrison, W.H., Luanne, L.R., Franklin, E.B., David, S.H., Kevin, B.H., “Corn stover fraction and bioenergy”,..., 129, 104-116 (2006).
26 Zaldivar, J., Martinez, A., Ingram, L.O., “Effect of selected aldehydes on the growth and fermentation of ethanologenic”,.., 65, 24-33 (1999).
27 Zaldivar, J., Martinez, A., Ingram, L.O., “Effect of alcohol compounds found in hemicellulose hydrolysate on the growth and fermentation of ethanologenic”,.., 68, 524-530 (2000).
28 Wei, G.H., Yang, X., Fermentation Engineering, Science Press, Beijing (2008). (in Chinese)
2008-11-28,
2009-02-25.
the Agriculture Application Investigation and Improvement Item of New Countryside Construction and Promotion Project of the Bureau of Science and Technology in Xi’an (NC08005).
** To whom correspondence should be addressed. E-mail: fandaidi@nwu.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Design and Performance Analysis of Micro Proton Exchange Membrane Fuel Cells*
- Isolation of Cordyceps ophioglossoides L2 from Fruit Body and Optimization of Fermentation Conditions for Its Mycelial Growth*
- Simulating Surface Aeration Systems at Different Scale of Mixing Time*
- Kinetics of Reaction-Crystallization of Struvite in the Continuous Draft Tube Magma Type Crystallizers—Influence of Different Internal Hydrodynamics
- Preparation and Characterization of Tungsten-substituted Molybdophosphoric Acids and Catalytic Cyclodehydration of 1,4-Butanediol to Tetrahydrofuran*
- Facile Preparation of Danazol Nanoparticles by High-Gravity Anti-solvent Precipitation (HGAP) Method*
